Abstract: AB2O4-type spinels with low relative permittivity (εr) and high quality factor (Q × f) are crucial to high-speed signal propagation systems. In this work, Zn2+/Ge4+ co-doping to substitute Ga3+ in ZnGa2O4 was designed to lower the sintering temperature and adjust the thermal stability of resonance frequency simultaneously. Zn1+xGa2−2xGexO4 (0.1 ⩽ x ⩽ 0.5) ceramics were synthesised by the conventional solid-state method. Zn2+/Ge4+ co-substitution induced minimal variation in the macroscopical spinel structure, which effectively lowered the sintering temperature from 1385 to 1250 °C. All compositions crystallized in a normal spinel structure and exhibited dense microstructures and excellent microwave dielectric properties. The compositional dependent quality factor was related to the microstructural variation, being confirmed by Raman features. A composition with x = 0.3 shows the best dielectric properties with εr ≈ 10.09, Q × f ≈ 112,700 THz, and τf ≈ −75.6 ppm/°C. The negative τf value was further adjusted to be near-zero through the formation of composite ceramics with TiO2.
Keywords: ceramics; spinel; composition modulation; dielectric properties
1 Introduction
Microwave dielectric ceramics can meet the evergrowing requirements of wireless communication as primary functional materials for dielectric resonators and filters. Radars, mobile networking (4G and 5G), base stations, satellite transmission, and global positioning systems are only a few examples of applications where such dielectrics have been commercialized [1–3]. The last few decades have witnessed intensive development and commercialization of microwave dielectric ceramics and a lot of candidates with good dielectric performances (high relative permittivity εr, high quality factor Q × f, tunable temperature coefficient of resonance frequency τf) have been explored [4–6]. Unfortunately, the undesirable incompatibility between these three merits, however, restricts the range of possibilities for practical applications [7]. As a consequence, there is still a high demand ongoing for novel microwave dielectrics with optimized performances for the above-mentioned merits.
AB2O4 spinel materials have received intense attention and are now considered as attractive microwave dielectric materials due to their high quality factors, e.g., (Mg,Zn)Al2O4 (Q × f ≈ 85,100 and 56,300 GHz), Mg2TiO4 (Q × f ≈ 150,000 GHz), CoZnTiO4 (Q × f ≈ 97,500 GHz), etc. [8–11]. Ga-based spinels particularly have also been reported as promising materials for millimeter-wave devices due to their low permittivities and high quality factors. For example, MgGa2O4 has a low εr of 9.5, a Q × f of 117,000 GHz, and a near-zero τf of −4.0 ppm/℃, while the isostructural ZnGa2O4 possesses a large negative τf of −27 ppm/℃, which would severely restrict its commercial potential [12].
Different attempts have been conducted to improve the thermal drift of τf for ZnGa2O4. Rutile TiO2 (6.9 vol%) was proved to be effective to compensate for the τf of ZnGa2O4 to be 3 ppm/℃ [13]. Furthermore, cation substitution in either A- or B-site was found to simultaneously improve the thermal stability and increase the quality factor of ZnGa2O4. Mn2+ substitution in ZnGa2O4 tuned the τf value to be −12 ppm/℃ coupled by a twofold increase in Q × f value to 181,000 GHz, whereas Cu2+ substitution reduced the temperature stability of resonance frequency despite the obvious improvement in quality factor (by ~65% to 131,500 GHz) [14,15]. Such variations in dielectric performances should be strongly related to the cation distribution and occupation preference, which would determine the normal or inverse crystal structure of the spinel. Additionally, given that the ZnGa2O4 is a normal spinel while MgGa2O4 is a partial inverse spinel, the difference in dielectric properties between the two materials may be due to structural differences in the cation distribution in tetrahedra and octahedra sites. As a result, establishing a primary correlation between the cation distribution and dielectric properties in spinels is extremely important.
Keeping in view the above challenges, spinel ZnGa2O4 was selected as the parent material and Zn2+/Ge4+ co-substitution for Ga3+ was proposed to form solid solution of Zn1+xGa2−2xGexO4 with an aim to study the correlation among cation occupation, crystal structure, and microwave dielectric properties.
2 Experimental
2. 1 Ceramics fabrication
Analytically pure ZnO, Ga2O3, and GeO2 (99.99%; Guo-Yao Co., Ltd., China) were used as raw materials to synthesize a series of nominal compositions of Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics by the conventional solid-state reaction method. The raw materials were mixed using a ball milling for 6 h with ZrO2 balls as a grinding media at a speed of 300 rpm. The slurry was subsequently dried at 120℃ for 2 h followed by calcination at 1150℃ for 6 h. The calcined powders were re-milled, dried, and mixed with 5 wt% PVA, which was used as the binder. The mixed powders were then pressed into disks of 10 mm in diameter and 6 mm in height under a uniaxial pressure of 80 MPa. To extract the organic binder, the green samples were heated to 550℃ for 6 h at a rate of 1.5 ℃/min, followed by calcination in a temperature range of 1250–1340 for 6 h.
2. 2 Structural and dielectric measurements
The phase composition and purity were investigated by X-ray diffractometer (XRD; Cu Kα1, 1.54059 Å, Model X’ Pert PRO, PANalytical, Almelo, the Netherlands). The crystal structure of all compositions was identified by the Rietveld refinement and Raman spectrometer (DXR; Thermo Fisher Scientific, USA). The bulk density of the sintered samples was measured by the Archimedes’ principle. The microstructure of polished and thermally etched samples was observed using scanning electron microscopy (SEM; S4800, Hitachi, Tokyo, Japan). The etching temperature was 50 ℃ lower than the optimum sintering temperature.The microwave dielectric properties were measured using a network analyzer (N5230A, Agilent Co., Palo Alto, CA, USA) connected with a temperature chamber (Delta 9039, Delta Design, San Diego, CA, USA). τf values were calculated using the following equation:

where f1 and f2 represent the resonant frequency at T1 (25℃) and T2 (85℃), respectively. The room - temperature infrared reflectivity spectra were measured by a Bruker IFS 66v FT-IR spectrometer (Bruker Optics, Ettlingen, Germany).
3 Results and discussion
Figure 1 shows the room-temperature XRD patterns of the Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics calcined 1150 . ℃ All the observed peaks were indexed with the PDF card No. 86-0415 and the crystal structure could be assigned to the cubic spinel ZnGa2O4 with space group Fd3▔m . Furthermore, all compositions exhibited identical diffraction patterns with no evidence of the second phase, suggesting a single-phase solid solution formed in the whole compositional range. Another evidence that endorsed the formation of the solid solution was the slight shift to the lower angle of the diffraction peaks, as a representative in Fig. 1(b) for the enlarged profile of (311) peak (around 2θ = 36°). The variation in ionic radius between Ga3+ and the doped (Zn0.5Ge0.5)3+ was responsible for the peak shift [16] as Zn1+xGa2−2xGexO4 previously reported to form spinel solid solutions for x ranging from 0 to 0.5 [17].
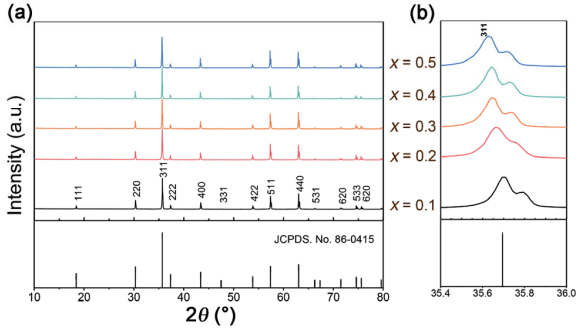
Fig. 1 (a) X-ray powder diffraction patterns of the Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics fired at 1150 ℃ and (b) amplified diffraction peak (311) around 2θ = 36°.
Rietveld refinement was conducted to further confirm the phase purity and crystal structure of the Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) samples and a structural model was built based on the crystal structure data of ZnGa2O4 [18]. A perspective view of the spinel structure of ZnGa2O4 is shown in Fig. 2, with the characteristic feature of bivalent Zn2+ occupying the tetrahedral sites and trivalent Ga3+ in the octahedral sites, featuring a normal spinel structure. Especially, for Rietveld refinement the doped (Zn0.5Ge0.5)3+ was fixed in the octahedral interstices. As shown in Figs. 3(a)–3(e), good matching between the calculated and the observed profiles indicates the structural model’s reliability and yields the accuracy of fit indicators (as shown in Table 1). The bond length of Zn–O1 initially decreased with x increasing from 0.1 to 0.3 and then increased for the remaining composition. On the other hand, the length of Ga–O1 and angle of Zn–O1–Ga decreased with increasing x, indicating the effects of cation substitution on bond features. The refined cell parameter a and cell volume V are plotted in Fig. 3(f) as a function of composition with 0.1 ≤ x ≤ 0.5 and saw a linear increase with increasing x (from 0.1 to 0.5) which is also validated by Vegard’s law [19]. These variations also proves the solid solution formation and can further be explained by the larger average ion radii of Zn2+ (R = 0.74 Å, CN = 6) and Ge4+ (R = 0.53 Å, CN = 6) (with an average radius of 0.635 Å) than the Ga3+ (R = 0.62 Å, CN = 6) [16].
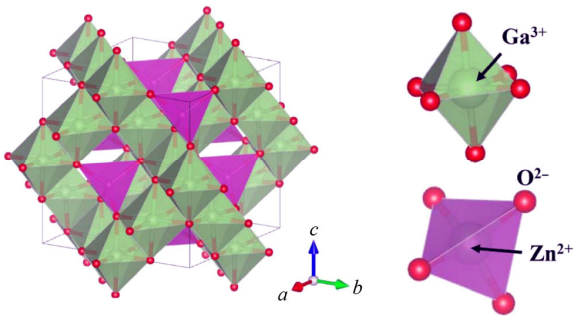
Fig. 2 A perspective view of the crystal structure of spinel ZnGa2O4.
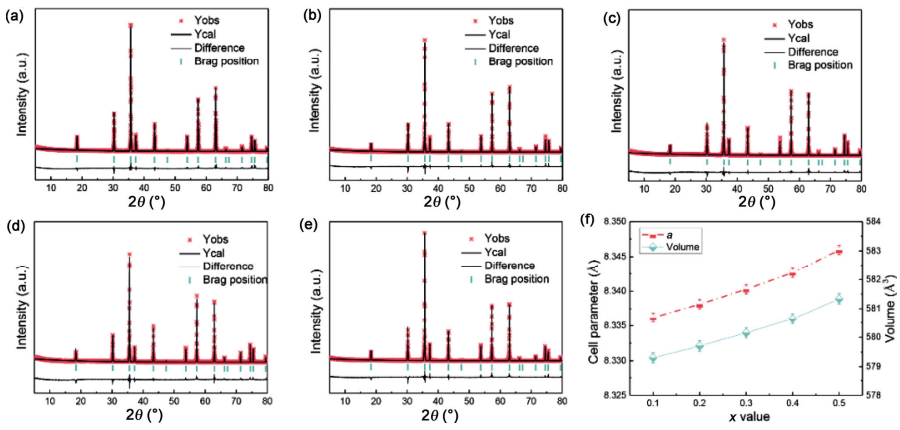
Fig. 3 (a–e) Rietveld refinement patterns for Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics (the red cross symbol represents the measured data while the solid line represents the calculated ones) and (f) variation of lattice parameters as a function of x value.
To investigate the influence of sintering temperature on the densification of Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics, the temperature dependence of the bulk density was plotted and is shown in Fig. S1 in the Electronic Supplementary Material (ESM). A tendency variation similar to bond length was observed for bulk density with increasing sintering temperature and showed an initial increase followed by a subsequent decrease. The optimum sintering temperature and the corresponding bulk density are shown in Fig. 4(a) as a function of composition (x value). The bulk density gradually increased from 5.82 to 5.96 g/cm³, as predicted from the calculated theoretical density. Notably, the optimum sintering temperature decreased significantly, although the relative density increased slightly from 94.5% at x = 0.1 to 97.2% at x = 0.5. Because of the low melt point of GeO2 (~1115℃), the addition of Zn/Ge improved the sintering characteristic of Zn1+xGa2−2xGexO4. Similar phenomena have previously been reported in Zn2GeO4 systems [20].
Table 1 Refined crystal structure parameters of Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5)
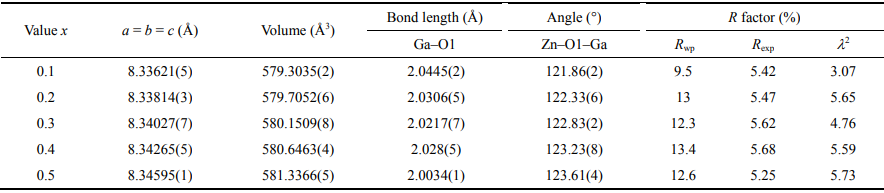
Figure 4(b) shows the compositional variation in the dielectric permittivity of Zn1+xGa2−2xGexO4 with increasing x from 0.1 to 0.5. The relative permittivity increased linearly from 9.78 at x = 0.1 to 10.42 at x = 0.5. Based on the Clausius–Mossotti equation (ε ={3Vm+ 8παD}/{3Vm+ 4παD})), the relative permittivity depends upon the total molecular polarizability (α) and unit cell volume (V) and is directly proportional to the polarizability density (α/V) [21,22]. The theoretical relative permittivity estimated by the Clausius–Mossotti equation was ~7.6% higher than the measured values, as shown in Fig. 4(b). After porosity corrected based on the Bosman and Having’s equation εcorr = εr × (1 + 1.5p) where p represents the fractional porosity, the deviation of the corrected permittivities from the estimated values was in the range of 3.6%–6.4% [23–26]. This result indicates that porosity (or density) has a direct influence on the permittivity of the material.
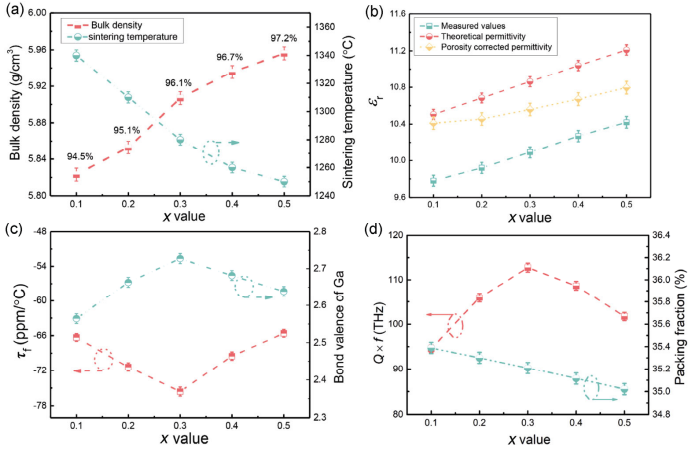
Fig. 4 Compositional dependence of bulk density and microwave dielectric properties of Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics sintered at their optimum temperature.
The τf values of the Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5) ceramics sintered at their relative optimum temperatures are shown in Fig. 4(c). The τf values decreased initially from −66.3 to −75.6 ppm/℃ followed by an increase to −65.6 ppm/℃ at x = 0.5. The Ga–O bond valence in the octahedron was calculated by the following equations [27]:
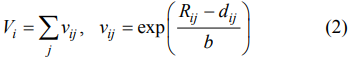
where Rij represents the bond valence parameter which has been summarized by Brown and Altermatt [28], dij is the length of a bond between atoms i and j, and b is a universal constant (0.37 Å). The variation in bond valence (x from 0.1 to 0.3) followed a pattern, opposite to τf value in which the bond valence initially increased followed by a decrease. The various bond valences correspond to the different atom interactions [29] and a higher valence means stronger interaction and higher band energy. In the present system, the increase in Ga–O bands resulted in the increasing resorting force, which recovered the titling of the octahedron, and thus a decrease in τf value.
In contrast to the permittivity, the quality factor exhibited an increasing trend with increasing Zn/Ge doping content (x value) from 0.1 to 0.3 and then declined. As reported by Kim et al. [30], Yin et al. [31], and Li et al. [32], the quality factor can be intrinsically predicted by packing fraction. A higher packing fraction signifies a smaller space for ion vibration, which leads to lower dielectric losses. The calculated packing fraction for Zn1+xGa2−2xGexO4 shows a decrease with the variation of x. Intrinsically, the quality factors were expected to exhibit a decreasing trend. The observed deviation suggested that the intrinsic packing fraction was not the only dominant influencing factor but other extrinsic factors, e.g., pores, grain size, cation ordering, etc., also play a part [6,33–35].
To investigate the underlying factors influencing the quality factor, the grain morphology and size distribution were studied. As shown in Fig. 5, the polished and thermal etched surfaces for all compositions are shown. Dense microstructures consisted of uniform grains and defined grain boundaries and a few visible pores, were observed. All compositions exhibited similar grain size distribution with average grain size in the range of 4.6–5.2 μm (Fig. 5(f)). Along with the higher relative density (94%–98%), the extrinsic contribution from density, pores, and grain size on the quality factor can be ruled out in relation to their dependency on the quality factor.
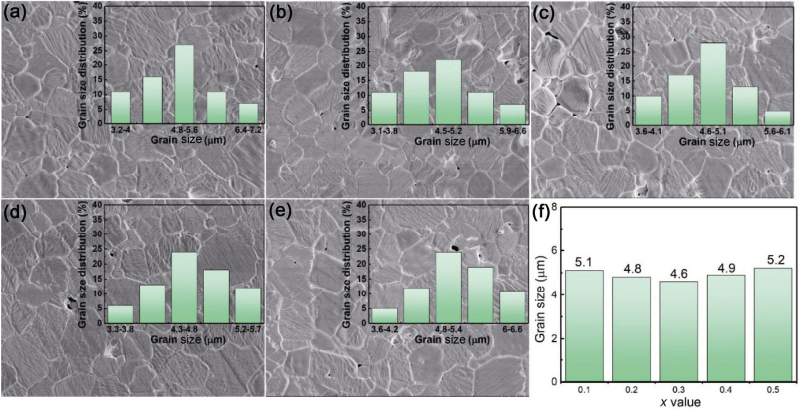
Fig. 5 Polished and etched microstructure of Zn1+xGa2−2xGexO4 ceramics: (a) x = 0.1 sintered at 1340℃ , (b) x = 0.2 sintered at 1310℃ , (c) x = 0.3 sintered at 1280℃ , (d) x = 0.4 sintered at 1260℃ , and (e) x = 0.5 sintered at 1250℃ .
Raman spectroscopy has been recognized to be an effective approach to correlate the lattice vibrational modes with dielectric properties, especially in those materials with cation ordering, e.g., Ba(Mg1/3Ta2/3)O3 complex perovskite [36] and A2MgTeO6 (A = Ca, Sr, Ba) double perovskites [37]. To explore the structural correlation of the microwave dielectric properties for Zn1+xGa2−2xGexO4 spinel, Raman spectroscopy was utilized at room temperature for all compositions.
According to the space group theory, ZnGa2O4 is a cubic spinel with space group Fd3▔m and has 14 atoms in its primitive unit cell. The irreducible representations can be given as [38,39]:
Г = A1g(R) + Eg(R) + T1g +3T2g(R) + 2A2u + 2Eu+ 5T1u(IR) + 2T2u (3)
where R and IR represent the Raman and infrared active modes, respectively. Among them, T1g, A2u, Eu, and T2u modes are silent [40,41]. Generally, five modes (Eg, 3T2g, A1g) with the first-order could be detected in the spine structure. As shown in Fig. 6(a), similar Raman profiles were observed and after Gaussia–Lnorentzian fitting (Figs. 6(a) and 6(d) as a representative) only three first-order modes could be recorded, which were positioned at 463 cm-1 (T12g), 610 cm-1 (T22g), and 712 cm-1 (A1g) [42]. The T12g mode was related to the Zn–O symmetric bending vibrations. The A1g mode and T22g modes were assigned to the symmetric and asymmetric stretching vibrations of ZnO4 tetrahedron [40]. The relative intensity of T12g and T22g gradually decreased with the increasing Zn/Ge doping, as displayed in Fig. 6(a). A similar phenomenon has been observed in Mg2(Ti1−xSnx)O4, which is caused by the different ion radio between Ti4+ and Sn4+ [43]. In this work, the effective ionic radius of (Zn0.5Ge0.5)3+ was larger than Ga3+, which was responsible for the variation in the Raman intensity. Additionally, it should be noted that the characteristic mode (A*1g, ~673 cm-1) for the direct recognition of an inverted spinel was silent for all Zn1+xGa2−2xGexO4 ceramics, confirming a stable normal spinel structure [43].
Figure 6(d) shows the Raman shift and FWHM (full width at half maximum) of A1g mode as a function of x value. The A1g mode had a blue shift while the FWHM gradually decreased to x = 0.3 initially followed by an increment to x = 0.5. The blue shift indicated the vibration phonon energy increased which limited the movement of the ions and decreased the polarizability. The FWHM was inversely correlated to the lifetime of phonon, and lower FWHM values mean the longer lifetime of phonon and less interaction with other phonons. These interactions can increase the intrinsic dielectric loss due to energy consumption. Thus, decreasing FWHM values are beneficial to improve the quality factor.
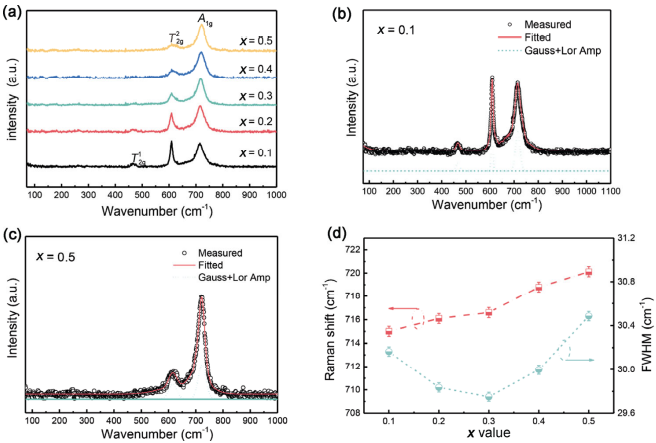
Fig. 6 (a) Room temperature Raman spectra of the Zn1+xGa2−2xGexO4 (0.1 ≤ x ≤ 0.5), (b, c) fitted results of x = 0.1 and 0.5, respectively, and (d) change of Raman shift and FWHM as a function of x value.
The fitted and measured values of IR reflectivity are shown in Fig. 7(a) while the real and imaginary parts of the complex permittivity are shown in Fig. 7(b). For ceramic with x = 0.3, there were 7 modes used and the detailed information on the fitted parameters is listed in Table 2. Out of those 7 modes, the modes at 303.46 and 424.26 cm-1 had a primary contribution to the dielectric properties. Based on the fitted parameters, the calculated permittivity in optical range (ε∞) was 1.92 while in the microwave frequency range, the permittivity was 9.22. These values were significantly lower than the measured values (εr = 10.04 for x = 0.3), which could be attributed to the extrinsic contributions stemming from the defects [44]. Compared to the total permittivity, the contributions of the optical permittivity were only 19.1%, suggesting that the primary contributions to the microwave dielectric properties were dominated by ionic polarization compared to electronic polarization [45]. On the other hand, the theoretical quality factor was fitted to be 153,000 GHz, which was much higher than the measured Q × f value (112,700 GHz). The deviation can be related to the processing parameters, e.g., the density, pore, and grain size. However, the fitting results indicate that there is still a wide space for dielectric properties optimization in the Zn1+xGa2−2xGexO4 ceramic system.
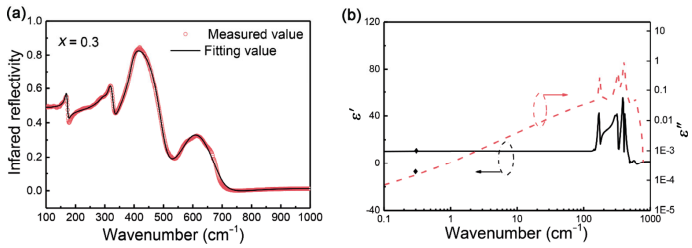
Fig. 7 (a) Measured and calculated infrared reflectivity spectra and (b) real and imaginary parts of the complex responses obtained from the fitting of the IR reflectivity spectra.
Table 2 Phonon parameters obtained from the fitting of the infrared reflectivity spectra of the x = 0.3 (Zn1.3Ga1.4Ge0.3O4) ceramic
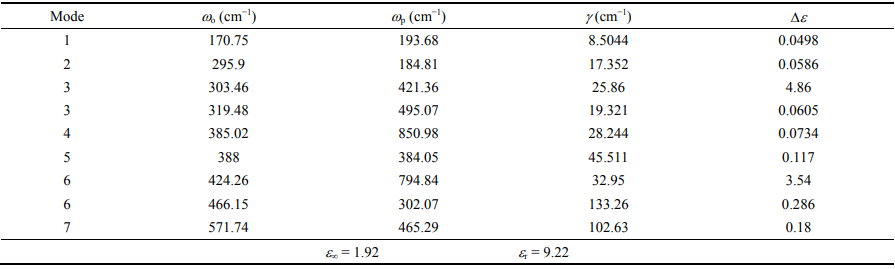
To further investigate the intrinsic dielectric properties of Zn1+xGa2−2xGexO4 ceramics, the IR reflectivity spectrum of x = 0.3 sample as a representative was fitted using a classical harmonic oscillator modeled as follows [46,47]:
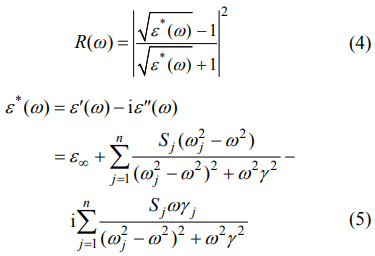
where n represents the order of transverse polarphonon modes; ωj, Sj, and γj are the frequency, strength, and damping constant of the j-th mode, respectively; and ε∞ is the relative permittivity caused by electronic polarization. When ω << ωj, Eqs. (6) and (7) are reasonable:
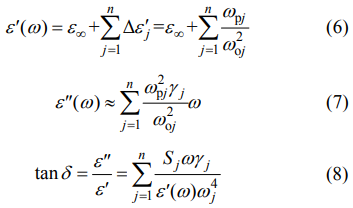
Table 3 shows some spinel-type ceramics with their optimum sintering temperatures and microwave dielectric properties. Compared with the parent ZnGa2O4, the Q × f value increased by 19.1% after Zn/Ge co-doping, whereas the temperature stability of the resonance declined. Commonly, the listed spinels possess low relative permittivity and high quality factor, rendering their potential applications in high-speed signal propagation fields. However, the negative τf values signify minor thermal drift of resonance frequency, which largely imposes a limit to their commercial promotion. To adjust the τf values of the present spinels, we have adopted TiO2 as an τf compensator to form composite ceramics with Zn1+xGa2−2xGexO4. As represent, a series of compositions of (1−x)Zn1.3Ga1.4Ge0.3O4 (x = 0.3)−xTiO2 (x = 0.1, 0.2, 0.3, 0.4) are arranged.
Table 3 Sintering temperature and microwave dielectric properties of some spinel-type ceramics
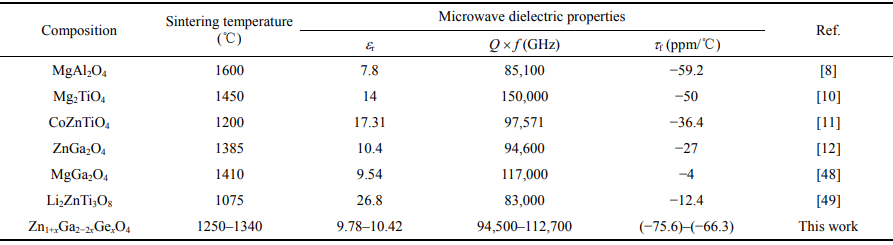
Table 4 Microwave dielectric properties of (1−y)Zn1.3Ga1.4Ge0.3O4–yTiO2 ceramics sintered at their optimum sintering temperature
Figure 8 shows the XRD, SEM, and EDS recorded on the representative 0.7Zn1.3Ga1.4Ge0.3O4–0.3TiO2 composite fired at 1270℃ for 6 h. XRD profiles demonstrate only the diffraction peaks from the spinel phase and rutile (JCPDS#01-1292), with no other phase detected, suggesting no chemical reaction occurred between them. SEM images featured distinct grains with different sizes, morphologies, and elemental contrasts. Upon conducting EDS analysis on different grains, the small bright grains (marked with #) were rich in Ti and Zn/Ge/Ga were not identified. As a result, it was concluded that the marked grains were TiO2. These results provide evidence for the chemical compatibility between TiO2 and Zn1+xGa2−2xGexO4, which is a prerequisite for property modulation.
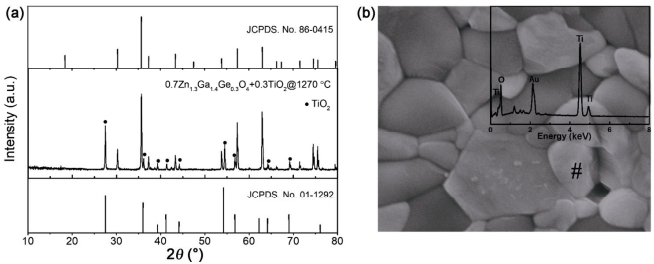
Fig. 8 (a) XRD patterns and (b) SEM of 0.7Zn1.3Ga1.4Ge0.3O4–0.3TiO2 ceramic sintered at 1270℃ for 6 h (the EDS analysis of TiO2 is shown in the inset).
As expected, TiO2 successfully tuned the negative τf value of the representative x = 0.3 sample to close to zero (+6.1 ppm/℃) when adding 30 mol% TiO2. Meanwhile, with the increasing TiO2 content, the relative permittivity gradually increased to 17.7 while the quality factor decreased to 97,200 GHz, which was reasonable in consideration of the high relative permittivity and low quality factor of TiO2 ceramic (εr ≈ 105 and Q × f ≈ 46,000 GHz) [50].
4 Conclusions
In summary, lowered sintering temperature and improved dielectric properties are simultaneously achieved by introducing Zn2+/Ge4+ to substitute Ga3+ in the ZnGa2O4 system. Solid solutions with a normal spinel structure were successfully synthesized in the whole composition range with 0.1 ≤ x ≤ 0.5. Combined microwave dielectric properties with εr ≈ 10.09, Q × f ≈ 112,700 GHz, and τf ≈ −75.6 ppm/℃ were obtained in a composition with x = 0.3 when sintered at 1280℃ . The thermal stability of resonance frequency was achieved through the formation of ceramic composite with TiO2. A nearzero τf value was achieved in x = 0.3 sample with 30 mol% TiO2 and achieved dielectric values of εr ≈ 14.7 and Q × f ≈ 101,800 GHz. All the merits demonstrated that the Zn1+xGa2−2xGexO4 ceramic is a promising candidate for high-speed signal propagation applications.
References: Omitted
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.