Abstract: Rare-earth tantalates and niobates (RE3TaO7 and RE3NbO7) have been considered as promising candidate thermal barrier coating (TBC) materials in next generation gas-turbine engines due to their ultra-low thermal conductivity and better thermal stability than yttria-stabilized zirconia (YSZ). However, the low Vickers hardness and toughness are the main shortcomings of RE3TaO7 and RE3NbO7 that limit their applications as TBC materials. To increase the hardness, high entropy (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 are designed and synthesized in this study. These high entropy ceramics exhibit high Vickers hardness (10.9–12.0 GPa), close thermal expansion coefficients to that of single-principal-component RE3TaO7 and RE3NbO7 (7.9×10-6–10.8×10-6 ℃–1 at room temperature), good phase stability, and good chemical compatibility with thermally grown Al2O3, which make them promising for applications as candidate TBC materials.
Keywords: high entropy ceramics; defective fluorite structure; rare-earth niobates/tantalates; thermal barrier coating material
1 Introduction
Thermal barrier coatings (TBCs) have widely been used in the gas-turbine engines to improve the energy efficiency and protect the hot structure components from foreign particle impact, water vapor, and molten salt corrosion [1–6]. However, the harsh service conditions immensely restrict the selection of TBC materials. Generally, there are several basic requirements for the selection of TBC materials, including high thermal stability, low thermal conductivity, high thermal expansion coefficient matching with the metal substrate, good corrosion resistance, and sluggish sintering rate [7–9].
Currently, the state-of-the-art TBC material is yttria-stabilized zirconia (YSZ) due to its outstanding mechanical and thermal properties, such as high strength and toughness, high melting point, close thermal expansion coefficient to that of metal substrate, and low thermal conductivity [10–12]. The major disadvantage of YSZ is the phase transformation, which leads to the abrupt volume change and coating cracking at higher temperatures such that the long-term service temperature is restricted to below 1200 ℃ [13]. Moreover, the severe sintering and high oxygen conductivity also limit its application in higher operation temperatures [7,14]. In order to further increase the operating temperatures of gas-turbine engines, several types of new TBC materials such as RE2Zr2O7, RE3NbO7, and RE3TaO7 (RE = rare earth element) with better thermal stability and lower thermal conductivity have been proposed [15–17]. In particular, rare-earth tantalates (RE3TaO7) and rare-earth niobates (RE3NbO7) have attracted enormous attention in recent years. The crystal structures of RE3TaO7 and RE3NbO7 are variable with the change of the containing REs. For RE3TaO7, when RE is La–Dy or Y, the crystal structure is ordered orthorhombic, while the crystal structure of the rest (RE is Ho–Lu) is defective fluorite, which is analogous to that of Y2Zr2O7 [18,19]. Similarly, with the decrease of ionic radius of REs, the crystal structure of RE3NbO7 changes from orthorhombic weberite (La–Tb) to defective fluorite (Dy–Lu, Y) [19–21]. More importantly, due to the combination of many intriguing properties, such as good thermal stability, large thermal expansion coefficients, simple crystal structure but extremely low thermal conductivity, RE3TaO7 and RE3NbO7 have been considered as promising TBC materials for higher temperature applications [16,17]. Nevertheless, previous studies indicate that the mechanical properties of RE3TaO7 and RE3NbO7, such as Vickers hardness, are significantly lower than those of YSZ [17,22–24]. Moreover, some compounds such as Sm3TaO7 and Sm3NbO7 exhibit phase transition with an abrupt volume change at 942 and 817 ℃, respectively, which is unfavorable to their application as TBC materials [22,25].
It is well known that the pursuits of superior performance, for instance, the combination of higher strength, good phase stability, lower thermal conductivity, and lower sintering rate are of critical interest for developing novel TBC materials. Recently, a new class of ceramics containing multi-principal elements have attracted growing interest, which are known as high entropy ceramics (HECs) because of their high configurational entropy [26–28]. Compared with the single principal-component ceramics, HECs exhibit fascinating properties like lower thermal conductivity, sluggish grain growth rate, better water-vapor resistance, and tunable thermal expansion coefficient [29–36]. The unique properties of HECs indicate that there is a new window for developing TBC materials, i.e., designing and synthesizing high entropy (HE) TBC materials with superior performance.
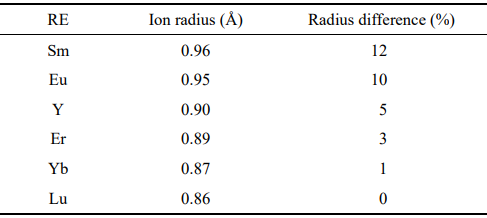
To improve the properties of RE3TaO7 and RE3NbO7 for thermal barrier application and overcome the possible phase transition, HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7, i.e., (Y1/3Yb1/3Er1/3)3NbO7, (Y1/3Yb1/3Er1/3)3TaO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7, are designed and successfully synthesized in this study. The choose of these REs is due to the following reasons. Firstly, the compounds containing these REs possess similar crystal structures. Secondly, the difference of ion radius of these REs is small (< 15%, Table 1), which warrants the easy formation of phase-pure solid solution. Moreover, the selecting compounds (such as Y3TaO7, Y3NbO7, Er3NbO7, and so on) have been studied, which makes the comparison of the properties of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 with them convenient. The phase composition, microstructure, Vickers hardness, thermal expansion coefficients, and chemical compatibility with Al2O3 are investigated. The high hardness, better phase stability, and good chemical compatibility with Al2O3 indicate that these new types of HECs are promising as high-performance TBC materials.
Table 1 Ion radius and radius difference of the selecting REs for the design of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 (the data are obtained from the database of Materials studio program. © Accelrys Inc., San Diego, USA, 2014)
2 Experimental
HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 powders were synthesized by the solid-state reaction method. Rare-earth oxides, tantalum oxide, and niobium oxide powders (99.9% purity; HWRK Chem. Co., Ltd., Beijing, China) were mixed in the stoichiometric ratio of required HE compounds. In particular, the molar ratio of raw materials is Y2O3 : Yb2O3 : Er2O3 : Ta2O3 = 1 : 1 : 1 : 1 for (Y1/3Yb1/3Er1/3)3TaO7, Y2O3 : Yb2O3 : Er2O3 : Nb2O3 = 1 : 1 : 1 : 1 for (Y1/3Yb1/3Er1/3)3NbO7, and Sm2O3 : Eu2O3 : Y2O3 : Yb2O3 : Lu2O3 : Er2O3 : Ta2O3 : Nb2O3 = 1 : 1 : 1 : 1 : 1 : 1 : 1 : 1 for (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7, respectively. After that, the mixed powders were ball-milled in ethyl alcohol with agate balls for 8 h. Then the obtained mixed slurry was dried at 70 ℃ for 12 h and ground by an agate mortar to break the agglomeration. The obtained powders were compacted under a uniaxial pressure of 40 MPa to make green bodies and then heated at 1450 ℃ for 2 h. Finally, the as-heated compacts were broken and ball-milled for 12 h before drying in a vacuum oven at room temperature. The final products were screened by a 300-mesh sieve to filter out the coarse particles. For comparison, the single-principal-component Y3TaO7 and Y3NbO7 powders were also synthesized in the same experimental conditions.
The phase composition of as-synthesized powders was analyzed by an X-ray diffractometer (XRD, D8 Advanced, Bruker, Germany) using Cu Kα radiation (λ = 1.5406 Å) with a step size of 0.02° at a scanning rate of 2 (°)/min. The lattice parameters of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 were refined by the Rietveld method (TOPAS, Bruker Corp., Karlsruhe, Germany).
Dense HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 bulks were prepared by using a spark plasma sintering (SPS) apparatus (SPS-20T-6-IV, Shanghai Chenhua Science and Technology Co., Ltd., China) at 1650 ℃ for 4 min under a pressure of 40 MPa. Details of the preparation process were reported in our previous studies [34,36]. After sintering, the surfaces of as-sintered compacts were ground by diamond to remove the carburized layer. The density of as-sintered compacts was measured by Archimede’s method. The phase composition of bulk compacts was analyzed by XRD. The microstructure and element distribution of bulk samples were investigated by a scanning electron microscope (SEM, Apollo300, CamScan, Cambridge, UK) with the attached energy dispersive X-ray spectroscopic (EDS) system (EDS Inca X-Max 80T, Oxford, UK). Before SEM observation, the samples were polished by #2000 SiC sand paper and thermally etched at 1400 ℃ for 2 h to make the grain boundary clear.
Vickers hardness measurement was performed by a micro-hardness tester (HXD-1000TMC/LCD, Shanghai Taiming, China) at a load of 9.8 N with a dwell time of
15 s. The linear thermal expansion coefficients were measured by an optical dilatometer (Misura ODHT 1600-50, Expert System Solutions, Italy) from room temperature to 1200 ℃ using the samples with a dimension of 3 mm × 4 mm × 15 mm. Before the test, the samples were polished by #2000 SiC sand paper and then chamfered at one end of the length. The length change of the samples (ΔL) with temperature (T) was recorded and the thermal expansion coefficient (α) was calculated by Eq. (1):
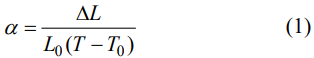
where L0 is the length of the sample at room temperature and T0 is room temperature.
Good chemical compatibility with thermally grown Al2O3 (TGO) is critical for TBC materials. In order to investigate the chemical compatibility between HE RE3TaO7/RE3NbO7/RE3(Nb1/2Ta1/2)O7 and Al2O3, the as-synthesized HE powders were mixed with α-Al2O3 powders (99.9% purity; HWRK Chem. Co., Ltd., Beijing, China) in a mass ratio of 1 : 1 by ball-milling, and then annealed at different temperatures for 2 h. For comparison, the chemical compatibility between Y3TaO7, Y3NbO7, and α-Al2O3 was also investigated by the same method. The phase composition of the annealed products was investigated by XRD.
3 Results and discussion
3. 1 Phase composition and microstructure
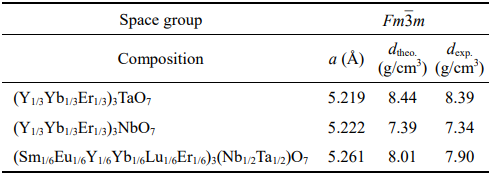
Figure 1 shows the XRD patterns of the as-synthesized Y3TaO7, Y3NbO7, and HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 powders. It can be seen that all the as-synthesized powders are phase-pure. The crystal structures of (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/6Ta1/6)O7 are defective fluorite despite that Y3TaO7 exhibits an orthorhombic structure. Interestingly, the intensities of the diffraction peaks from all reflections of (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 are significantly lower than those of the others. In addition, the widths of the peaks of (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 are broadened. This is one of the main characteristics of high entropy materials and can be explained by the intrinsic lattice distortion caused by the addition of multi-principal elements with different atomic sizes, which leads to the increase of the atomic plane roughness and the decrease of the diffraction peak intensity [37]. Based on the XRD patterns in Fig. 1, the lattice parameters of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 were refined and their theoretical densities were calculated, which are depicted in Table 2. The refined lattice parameter of (Y1/3Yb1/3Er1/3)3NbO7 is close to that of (Y1/3Yb1/3Er1/3)3TaO7. However, due to the addition of more multi-principal elements with different sizes, (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 exhibits severer lattice distortion and possesses a larger lattice parameter. Correspondingly, the theoretical densities of three HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 were calculated (Table 2).
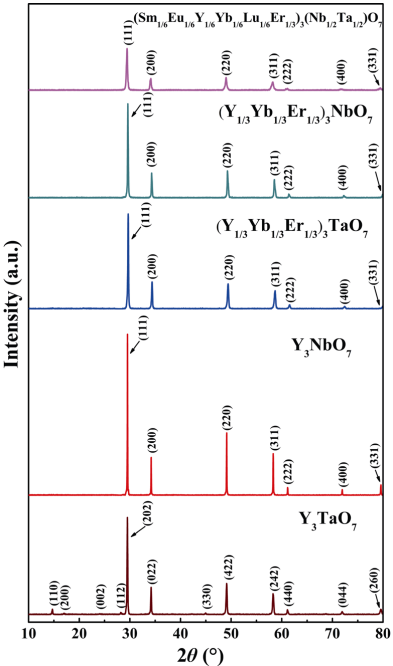
Fig. 1 XRD patterns of the as-synthesized Y3TaO7, Y3NbO7, and HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 powders.
Table 2 Refined lattice parameters of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 together with the calculated theoretical densities (dtheo.) and the experimental measured densities (dexp.)
In order to investigate the mechanical and thermal properties of HE RE3TaO7 , RE3NbO7 , and RE3(Nb1/2Ta1/2)O7, it is essential to prepare dense bulk compacts. Figure 2 exhibits the XRD patterns of the bulk HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 compacts prepared by SPS method. Clearly, the phase compositions of all three compacts are unchanged and no new phase can be detected, indicating that the HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 have good thermal stability. The SEM images of the bulk HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 compacts after thermal etching at 1400 ℃ for 2 h and the corresponding EDS mappings of the containing multi-principal metal elements are shown in Fig. 3. No pores and cracks can be observed at the surface of all three bulk materials, indicating that the as-sintered bulk compacts are near fully dense. The densities of (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 measured by Archimede’s method are depicted in Table 2. Clearly, the relative densities of all the samples are about 99%. Moreover, all the bulk compacts present equiaxed grains, but the average grain size of (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 is evidently smaller than those of (Y1/3Yb1/3Er1/3)3TaO7 and (Y1/3Yb1/3Er1/3)3NbO7 (Table 3). The grain size difference of three near fully dense materials can be explained by the sluggish diffusion effect of high entropy materials. The lattice distortion induced by addition of multi-principal elements hinders the atomic movement and effective diffusion of the atoms and thus makes the grain growth rate slower [38]. The EDS mappings indicate that all the containing principal metal elements of three bulk materials are evenly distributed and thus the homogeneous solid solutions are formed.
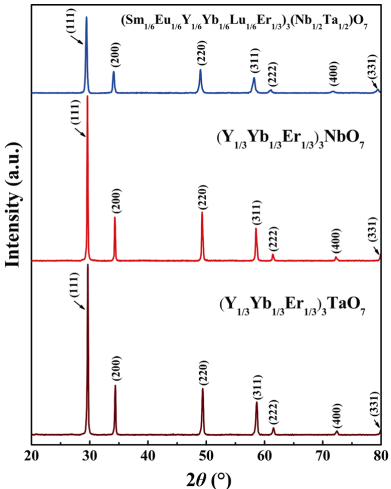
Fig. 2 XRD patterns of the bulk HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 compacts prepared by SPS method.
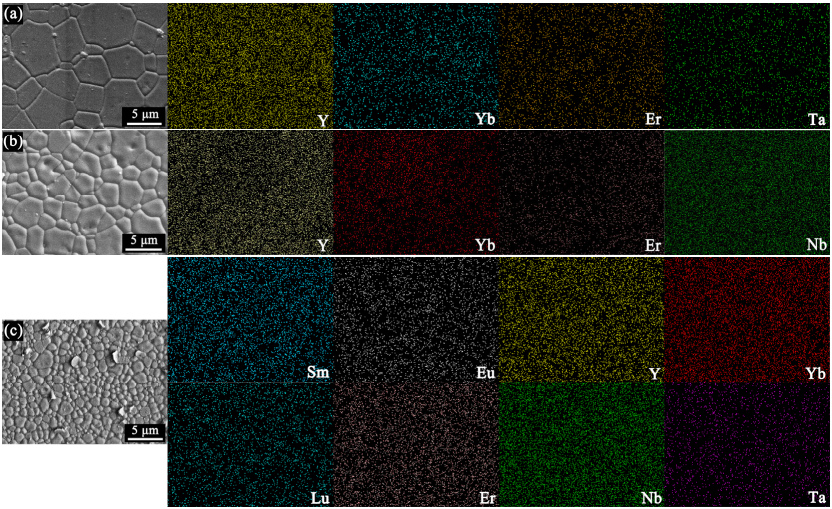
Fig. 3 SEM images and the corresponding EDS mappings of the containing multi-principal metal elements of the bulk HE
RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 compacts after thermal etching at 1400 ℃ for 2 h : (a) (Y1/3Yb1/3Er1/3)3TaO7, (b)
(Y1/3Yb1/3Er1/3)3NbO7, and (c) (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7.
3. 2 Vickers hardness
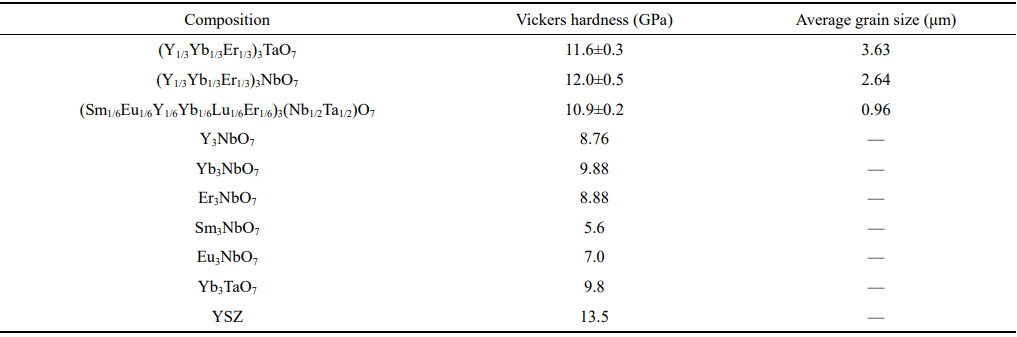
During the service, the thermal barrier coatings usually suffer from the corrosion caused by the impact of foreign particles in the fluid. Thus, Vickers hardness of TBC materials is an important parameter and high Vickers hardness can retard the impact corrosion rate of TBC materials. Table 3 exhibits the Vickers hardness of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 measured at a 9.8 N load and compared with the Vickers hardness of RE3NbO7, RE3TaO7, and YSZ [17,22–24]. Clearly, the Vickers hardness of (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 are close to that of YSZ, but higher than those of Y3NbO7, Yb3NbO7, Er3NbO7, Sm3NbO7, Eu3NbO7, and Yb3TaO7. The relative high hardness indicates that HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 may possess good impact corrosion resistance, which is beneficial to their application as thermal barrier coating materials.
Table 3 Vickers hardness and average grain size of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 measured at a 9.8 N load. The Vickers hardness of RE3NbO7, RE3TaO7, and YSZ are also included for comparison
3. 3 Thermal expansion coefficient (CTE) and phase stability
CTE is also a critical parameter to be taken into account. In order to diminish the thermal stress between TBCs and substrates, materials which have close CTE to that of the substrates are preferential for TBC applications. Figure 4 shows the linear CTEs of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 measured from room temperature to 1200 ℃. Based on Eq. (1), the linear CTEs of (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 from room temperature to 1200 ℃ are calculated to be 9.0×10-6, 10.8×10-6, and 7.9×10-6 ℃-1 , respectively. It is intriguing that CTE of (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3 (Nb1/2Ta1/2)O7 is much lower than those of (Y1/3Yb1/3Er1/3)3TaO7 and (Y1/3Yb1/3Er1/3)3NbO7. The result can be explained from two aspects. Firstly, the high entropy effect hinders the oscillation amplitude of constituting atoms by severe lattice distortion. Secondly, it is well known that the CTEs are related to the lattice energy. The average interatomic distance is related to the atomic interaction potential energy U(R) through Eq. (2) [39]:
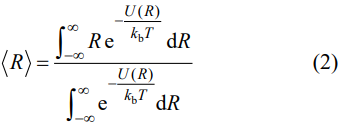
where kb is Boltzmann constant. In light of Eq. (2), materials with higher lattice energy usually exhibit lower CTEs. As reported in previous study of Chen et al. [23], the lattice energy of RE3NbO7 increases with the decreasing RE3+ ionic radius (La3+→Lu3+) due to the decrease of crystal structural order (ordered orthorhombic to defective fluorite). Thus the CTEs of RE3NbO7 increase with the decreasing RE3+ ionic radius. Since the ionic radius of Sm3+ and Eu3+ are larger than those of Y3+, Yb3+, and Er3+ (Table 1), the CTEs of the compounds containing Sm3+ and Eu3+ are smaller than those containing only Y3+, Yb3+, and Er3+. Thus the lower CTE of (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 may be explained by the high entropy effect which hinders the oscillation amplitude of constitute atoms by severe lattice distortion and the high lattice energy due to the addition of the REs with large ionic radius. Furthermore, the thermal expansion curves of all the samples are nearly linear and no phase transformation with abrupt volume change occurs in the measured temperature range. It is noteworthy that Sm3TaO7 and Sm3NbO7 exhibit abrupt volume change at 942 and 817 ℃, respectively, due to the phase transition [22,25]. Nevertheless, although samarium is selected as a principal element to be added into the solid solution, the abrupt volume change does not occur when (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 was heated from room temperature to 1200 ℃, which indicates that the phase transition is suppressed and all the HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 have good phase stability.
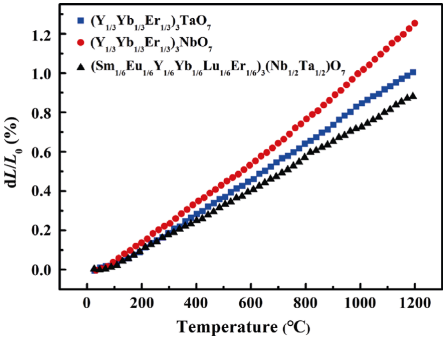
Fig. 4 Linear thermal expansion curves of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 measured from room temperature to 1200 ℃.
3. 4 Chemical compatibility with Al2O3
When gas-turbine engines operate at high temperatures, the interconnected porosity of ceramic coatings always allows the ingress of oxygen from the engine environment to the bond-coat layer, which results in the oxidation of bond-coat layer and the formation of TGO layer between the bond-coat layer and the ceramic top-coat layer [1]. Thus, TBC materials are required to possess good chemical compatibility with TGO. To investigate the chemical compatibility of HE RE3TaO7/RE3NbO7/RE3(Nb1/2Ta1/2)O7 with TGO, powder mixtures of HE RE3TaO7/RE3NbO7/RE3(Nb1/2Ta1/2)O7 and Al2O3 were heated to different temperatures for 2 h. Figure 5 shows the XRD patterns of the mixed powders of Y3TaO7, Y3NbO7, HE RE3TaO7, HE RE3NbO7, HE RE3(Nb1/2Ta1/2)O7, and α-Al2O3 after annealed at different temperatures for 2 h. As shown in Fig. 5(a), only α-Al2O3, Y3TaO7, Y3NbO7, and HE RE3TaO7, RE3NbO7, RE3(Nb1/2Ta1/2)O7 phases can be detected when annealed at 1200 ℃, which indicates that both Y3TaO7 and Y3NbO7, as well as HE RE3TaO7, RE3NbO7, RE3(Nb1/2Ta1/2)O7/Al2O3 have good chemical compatibility with α-Al2O3 at 1200 ℃ . The above results are consistent with those of Yang et al. [22], who reported the good chemical compatibility of RE3NbO7 with Al2O3 up to 1200 ℃. When the temperature increases to 1250 ℃, no new phase can be detected from the mixed powders of Y3TaO7/Al2O3, HE RE3TaO7/Al2O3, and RE3(Nb1/2Ta1/2)O7/Al2O3, which indicates that Y3TaO7, HE RE3TaO7, and RE3(Nb1/2Ta1/2)O7 remain good chemical compatibility with α-Al2O3 at 1250 ℃. However, Y3NbO7 and HE RE3NbO7 start to react with α-Al2O3 with the formation of RE3Al5O12 and RENbO4 (Fig. 5(b)). When annealed at 1300 ℃, all the samples react with Al2O3 and yield RE3Al5O12, RETaO4, RENbO4, and RE(Ta1/2Nb1/2)O4 (Fig. 5(c)). Since the maximum temperature for TGO is usually lower than 1200 ℃, we can conclude that HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 have good chemical compatibility with TGO. At higher operating temperatures, HE RE3TaO7 exhibits better chemical compatibility with TGO than HE RE3NbO7.
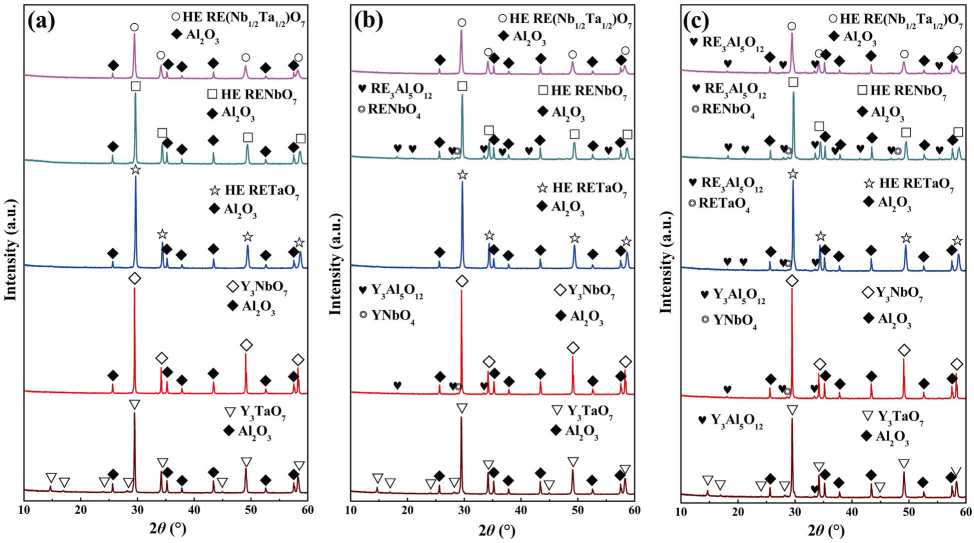
Fig. 5 XRD patterns of the mixed powders of Y3TaO7, Y3NbO7, HE RE3TaO7, HE RE3NbO7, HE RE3(Nb1/2Ta1/2)O7, and α-Al2O3 after annealing at different temperatures for 2 h: (a) 1200 ℃, (b) 1250 ℃, and (c) 1300 ℃.
4 Conclusions
In this study, novel HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7, i.e., (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 are designed and synthesized successfully. The combination of XRD, SEM, and EDS analysis indicates that the as-synthesized HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 are phase-pure with homogeneous distribution of metal elements. The Vickers hardness of HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 are in the range of 10.9–12.0 GPa, which are close to that of YSZ but significantly higher than those of singleprincipal-component RE3TaO7 and RE3NbO7. The thermal expansion coefficients of (Y1/3Yb1/3Er1/3)3TaO7, (Y1/3Yb1/3Er1/3)3NbO7, and (Sm1/6Eu1/6Y1/6Yb1/6Lu1/6Er1/6)3(Nb1/2Ta1/2)O7 from room temperature to 1200 ℃ are 9.0×10-6, 10.8×10-6, and 7.9×106 ℃-1, respectively. All the as-synthesized HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 exhibit good phase stability at least up to 1200 ℃. HE RE3TaO7 and RE3(Nb1/2Ta1/2)O7 show better chemical compatibility (without reaction up to 1250 ℃) with TGO than that of HE RE3NbO7 (without reaction up to 1200 ℃). The combination of high Vickers hardness, good phase stability, and good chemical compatibility with TGO indicates that HE RE3TaO7, RE3NbO7, and RE3(Nb1/2Ta1/2)O7 are suitable for applications as promising TBC materials.
References: omitted
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.