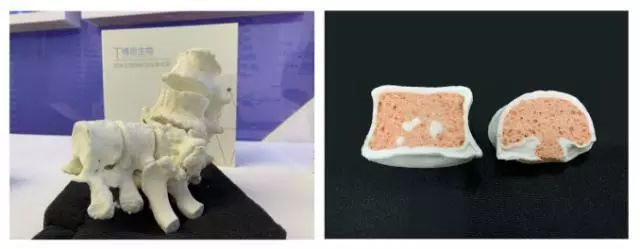
The biomaterials introduced before are mainly used to repair and replace human bones that have been traumatized, diseased, or congenital defects, or repair materials that can be permanently implanted in the body. Recently, an absorbable bioceramic material has been rapidly developed. This material is a form of tricalcium phosphate called "Synthos". This material is made into a porous bone block and implanted in the injured part of the human body. When the adjacent tissues proliferate and grow in the body of the tricalcium phosphate implant, the tricalcium phosphate can be slowly replaced by the regenerated bone of the animal. The process is called biodegradation process, so this type of material is also called biodegradable material or bioabsorbent material.

Tricalcium phosphate high artificial bone
1. Preparation of Tricalcium Phosphate
(1) Synthesis of tricalcium phosphate
Tricalcium phosphate [Ca3(PO4)2] is denoted as TCP. There are two forms of high-temperature α-phase and low-temperature β-phase, and β-phase is used as a bioceramic. TCP synthesis methods include high temperature solid-phase reaction method and wet method at room temperature, that is, aqueous solution reaction method. It is very difficult to synthesize pure β-TCP, no matter which method is used, there are more or less second phases such as α phase, HAP and CaO. The high and low temperature phase transition temperature is between 1120~1180℃, but the presence of impurities such as Mg and Na will have a greater impact on the temperature. The B-TCP powder synthesized by dry method is pulverized by ball milling for a long time and sintered under normal pressure to obtain a porous body. To prepare a high-density sintered body, a wet-synthesized micropowder must be used.
The raw materials for synthesizing TCP are similar to HAP. During the synthesis, the ingredients should be controlled to Ca/P=1.5. Wet synthesis is to slowly add the phosphorus-containing solution to the calcium-containing alkaline solution to form a white gel-like precipitate, which is amorphous phosphoric acid calcium. If 1%~2% ammonium sulfate is added to this gelatinous precipitate, and it is formed at 1100°C, pure TCP with close to the theoretical density can be obtained.
(2) Molding and firing process of porous calcium phosphate ceramics
Porous calcium phosphate ceramics are made of calcium phosphate powder as a raw material. After molding, they are fired at about 1150°C, with a heating rate of 90°C/h, and a temperature of 4.5h. The resulting sintered body is β-TCP single-phase.
The biodegradability of β-TCP is closely related to the firing temperature. Studies have shown that as the sintering temperature decreases, the solubility of β-TCP increases, so the sintering temperature should not exceed 1100°C. In addition, the porous network of pores is also conducive to material degradation.
Three molding methods can be used to prepare porous biodegradable materials:
① Add a pore-forming agent, such as paraffin, naphthalene, and other organic substances to the powder, and after pressing and molding, the pore-forming agent is burned out during the firing process, leaving uniform connected pores.
②Molding with foaming agent, that is, absorbing slurry with good organic foam molding. After drying, the ceramic blank is uniformly consolidated on the foam skeleton, and the foam is burned out during firing, leaving a porous ceramic body with a foam structure. This method requires that no harmful residues exist after the foam is lost on ignition, and requires a certain strength and good wetting and adsorption characteristics of the slurry.
③Using grouting molding method, namely molding with plaster mold. This method requires strict control of the water content of the plaster model. After grouting, the mold is dried in a constant temperature box with controllable humidity and temperature, for example, the temperature is raised to 80°C at a rate of 10°C/h, and the temperature is kept for 10 hours.
2. The structure and properties of tricalcium phosphate
As mentioned earlier, TCP has two crystal forms, the high-temperature α phase and the low-temperature β phase. α-TCP is a monoclinic crystal system with a density of 2.86g/cm3. β-TCP is a rhombohedron with a density of 3.07g/cm3. The chemical properties of β-TCP are similar to that of hydroxyapatite, and the solubility in water is slightly larger.
The compressive strength of the β-TCP sintered body is between 450 and 676 MPa, and the flexural strength of 137 to 156 MPa is similar to that of hydroxyapatite.
The solubility of TCP is greater than that of hydroxyapatite. Among them, β-TCP is approximately twice that of hydroxyapatite, and α-TCP is 10 times that of hydroxyapatite.
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.