Abstract
The study examined the feasibility of utilizing the mixture of ceramic sludge and roller kiln wastes, to produce low-cost ceramic-based membranes designated for use in wastewater treatment applications. In recent years, the treatment of wastewater contaminated with humic acid has posed signifcant challenges due to its complex nature and resistance to conventional treatment methods. To improve the physical, mechanical, and fltration qualities of the membranes, the study involved preparing them using a blend of fve distinct composition ratios of totally recycled ceramic sludge and roller kiln wastes, which were then sintered at temperatures ranging from 900℃ to 1300℃. The most efective membrane showed the best permeate fux and humic acid separation efciency for the wastewater samples when it was sintered at 1000℃ using only ceramic sludge waste. The produced membranes were thoroughly examined to reveal their structural and chemical characteristics. This confrmed the efective integration of functionalized multi-walled carbon nanotubes (f-MWCNTs) and their infuence on the membranes’ functionality. f-MWCNTs were added to the membrane’s surface via wet impregnation and drop casting methods. This resulted in a notable improvement in the membrane’s humic acid separation efciency, which increased to 92.61%, and the fux increased to 128.46 L/m2/h at a concentration of 100 mg L−1 as well. The opportunity to develop efective and environmentally sustainable ceramic membranes for water treatment using industrial ceramic wastes is highlighted by this study.
Keywords Waste recycling·Ceramic membrane·Carbon nanotubes·Surface modifcation ·Wastewater treatment
Introduction
Humic acids (HA) are pivotal components in terrestrial and aquatic ecosystems, originating from the decomposition of organic matter (Wang et al. 2016). While they play a crucial role in maintaining environmental equilibrium by aiding in nutrient cycling and improving soil structure, their presence in water systems presents considerable challenges. These challenges encompass alterations in water taste, odor, and color, as well as the promotion of biofouling in distribution systems (Dehkordi et al. 2015). Conventional methods for addressing these issues, such as photocatalytic degradation (PCD), often exhibit limitations. These include slow reaction kinetics and the potential formation of harmful by-products (Szymański et al. 2016; Ahmad et al. 2016). Consequently, there is an urgent need for more effective and efficient solutions. Among the emerging technologies, ceramic membrane filtration has emerged as a promising approach, demonstrating the effective removal of humic acid contaminants. This method not only addresses environmental concerns but also enhances water quality, positioning it as a focal point for advancing water treatment technologies (Xia et al. 2013).
The disposal of ceramic industrial waste, particularly from tile manufacturing processes, presents a significant environmental challenge due to the complexities associated with its accumulation and management (Anggono 2005). Roller kilns, integral to ceramic tile production for firing the product, are a critical contributor to this issue. While essential, these kilns raise environmental concerns, particularly regarding the need for regular maintenance, specifically the grinding of roller surfaces. This maintenance becomes imperative as rollers become contaminated with tile glaze or alkali salts, compromising kiln efficiency and operation (Ahmed et al. 2014). Additionally, ceramic tile sludge, an inevitable byproduct of wastewater treatment in ceramic tile factories, adds to the disposal conundrum. Comprising approximately 2% of the total product weight, this sludge is traditionally considered waste and its disposal poses both logistical and financial challenges (Roushdy 2019; Ramadan et al. 2008). However, recent research has highlighted a paradigm shift, considering this sludge not as waste but as a resource (Hegazy et al. 2012). Studies demonstrate its potential in manufacturing energy-efficient sintered tiles and blending it with glass proves to be a viable approach to meeting industry standards for tile strength, durability, or another specific characteristic (Nandi et al. 2015).
Ceramic membranes are widely used in water filtration and treatment processes because of their high permeability and cost-effectiveness (Jedidi et al. 2009; Ali et al. 2018). These membranes offer many advantages compared to polymeric ones, including stability at high temperatures, resistance to high pressure, and chemical resilience, especially in extreme pH conditions (Gomaa et al. 2024). As water scarcity becomes a critical concern globally, ceramic membranes have established themselves as crucial components in water treatment plants (Jedidi et al. 2011; Hanjra and Qureshi 2010). Moreover, ceramic membranes have demonstrated efficacy in various food processing industries, such as juice clarification, corn syrup filtration, raw rice wine production, and effluent treatment from fish processing plants (Vladisavljević et al. 2003; Emani et al. 2013; Almandoz et al. 2010; Li et al. 2010; Pérez-Gálvez et al. 2011). Recently, there has been a growing trend in using ceramic membranes made from low-cost materials or industrial waste streams for water treatment. This trend significantly reduces costs while maintaining superior properties and performance (Samadi et al. 2022; Guo et al. 2018; Nandi et al. 2008; Larbot et al. 2004). Thus, ceramic membrane usage holds promise for treating water polluted with humic acid (Xia et al. 2013). Ceramic membranes can be prepared by various techniques, including extrusion, pressing, and slip casting, all of which involve three fundamental steps are involved in membrane production. Firstly, a suitable binder is defined to be mixed with the ceramic powder. Then, it is shaped either by forming a suspension for slip casting, by adding sufficient water to form a paste for extrusion, or by molding the powder and pressing it. Finally, the shaped membranes are fired at high temperatures in a sintering process. Multilayer ceramic membranes can also be produced by coating the membrane support with suitable layers (by sol–gel, CVD, etc.) (Elma et al. 2012, 2015; Idakiev et al. 2005).
Wet impregnation, a technique used to enhance the adsorbent affinity and capacity of membrane surfaces, involves incorporating chemical species into the pores, followed by the introduction and drying of excess solvent (Abdel-Ghafar and Hamouda 2024; Girish 2018; Gray et al. 2018). Although this process aims to enhance adsorption capabilities, it can decrease the pore size and diameter due to the deposition of materials within the pores, which is commonly utilized in creating mesoporous materials (Yang et al. 2024). Additionally, impregnation facilitates the formation of an oxide film on the surface, leading to a decrease in regeneration temperature and an extension of the adsorbent's lifespan (Zhou et al. 2019; Liu et al. 2019). Alternatively, drop casting, a method for producing thin solid films by depositing liquid droplets onto a substrate, is simple, scalable, and cost-effective (Kumar et al. 2022). However, it is most effective for small-area coatings due to scalability limitations (Eslamian and Soltani-Kordshuli 2018).
This work introduces an innovative use of high alumina and silica waste materials from grinding roller kilns and ceramic sludge to fabricate stable ceramic membranes for wastewater treatment. The obtained membranes were utilized for humic acid removal with a high concentration of 100 mg L−1. The surface of the optimized membrane was modified using f-MWCNTs for enhancing the rejection rate.
The alumina and silica ceramic waste materials are collected from Rondy Ceramic company located at the industrial zone, kom Abu Rady city, Beni Suef Governorate, Egypt, in June 2022. The research activities carried out through this study at the laboratories of faculty of Earth Sciences, Beni-Suef University (Beni-Suef, Egypt), Central Metallurgical Research and Development Institute (CMRDI, Cairo, Egypt), and National Research Center (NRC, Cairo, Egypt) between 2022 and 2024.
Materials and methods
Materials
The primary precursors for the ceramic membrane preparation comprised ceramic sludge waste and the roller kiln’s hazardous fine waste. These materials were sourced locally from the Ceramic Rondy Company in the industrial zone of Kom Abu Rady city, Beni Suef Governorate, Upper Egypt. Additionally, polyvinyl alcohol (PVA, extra pure, molecular weight of 125,000), ethanol absolute (purity ≥ 99.9%, VWR, Germany), sulfuric acid (H2SO4, 98%, Adwic), and nitric acid (HNO3, 69–72%, Sigma-Aldrich) were procured and used as received. Furthermore, humic acid sodium salt with a technical grade of 50–60% (as humic acid) was obtained from Alfa Aesar Company in Germany. The pristine multi-walled carbon nanotube (p-MWCNTs) used in this study was procured from the Egyptian Petroleum Research Institute (EPRI) in Cairo, Egypt. All chemicals were of analytical grade and utilized as received without the need for further purification. De-ionized water was employed in all experimental procedures.
Characterization
The grind roller kiln and milled ceramic sludge wastes underwent several analyses. The chemical composition was determined using X-ray fluorescence (XRF) with the Malvern Panalytical Axios FAST simultaneous WDXRF Spectrometer, Netherlands. The mineralogical composition was determined using X-ray diffraction (XRD) with the Bruker D8 Discover diffractometer (Germany), operated at 40 kV and 40 mA, with a wavelength of 1.54A°. Thermal analyses (TGA–DTG) were performed using the THEMYS One +—Setaram Thermal Analyzer (France), with a temperature range of 35–1000℃ and a heating rate of 10℃/min, under N2 atmosphere. The particle size distribution (PSD) was investigated using the BT–2001(Liquid) Laser Particle Size Analyzer, conforming to ISO 13320 (ISO 2009). The true density of the powder was measured using the standard pycnometer test method (Density flask) according to ASTM B 311 (ASTM 2013), which is a very precise procedure for determining the density of powders, granules, and dispersions with poor flowability characteristics. Meanwhile, the bulk density was measured according to (ASTM 2009) which standardizes the measurement of bulk density (unit weight) and voids in aggregates. The bonding parameters and functional groups of the ceramic membranes were characterized using FT-IR spectroscopy. This analysis was carried out using a Bruker VERTEX 70v spectrometer within the wavenumber range of 400–4000 cm−1, with thin film samples measuring 2 cm in length and 1 cm in width. Pore size analysis of membranes was determined using a high-pressure mercury porosimeter, specifically the Micromeritics 9320, USA. Surface morphologies of the membranes were observed using a field emission scanning electron microscope (Quanta FEG250), Netherlands, attached to an EDX Unit (Energy Dispersive X-ray Analyses).
Preparation of the ceramic membranes
The ceramic sludge waste particles were milled and sieved to a standard aperture size of 80 µm to ensure uniformity. Following this, they were dried in an oven at 110 °C for 2 h. The roller kiln waste was utilized in its original form. Cylindrical disk samples, with a diameter of 50 mm and a thickness of approximately 10 mm, were formed using a pressing method. Mixtures of ceramic sludge waste and roller kiln waste powders, with varying weight ratios, were pressed with 15% PVA solution (by weight) in stainless steel molds using a laboratory hydraulic press under a uniaxial load of 25 MPa as outlined in Table 1. The membrane specimens were dried in two steps using a laboratory dryer: first at 80℃ for 8 h, then at 110℃ for another 6 h. The membrane specimens were then fired in a laboratory muffle furnace at five different temperatures (900, 1000, 1100, 1200, and 1300℃), each for a soaking time of 1 h (Table 1).
Table 1 Composition of the prepared ceramic membrane specimens

The heating rate was maintained at a constant 5 ℃/min. The firing technique employed in this work was single-slow firing, involving a gradual increase in temperature from room temperature to the required firing temperatures, followed by cooling to room temperature. Additionally, twenty cubic specimens, each measuring 5×5×5 cm, were prepared under identical manufacturing conditions to assess the mechanical properties of the ceramic membranes. The dimensions of the formed membranes were determined using a digital Vernier caliper, while their masses were measured using a digital analytical balance with a reading accuracy of up to 0.00001 g (AS X7, RADWAG). The obtained membranes are presented in Fig. S1. The physical and mechanical properties of the prepared membranes were determined according to standard test methods (ASTM 2014, 2003, 2011, 2006, 2016), as ascribed in the supplementary (SI).
Membrane testing and performance assessment
The lab-scale ceramic membrane filtration test unit used in this study, designed by CERAFILTEC, Germany, is a portable unit suitable for short-term filtration applications (Fig. 1a). It is equipped with a ceramic membrane testing cell featuring a 50 mm diameter, enabling the testing of small sample volumes. The process entails immersing a single test plate into a 10 L feeding water tank containing contaminated water, typically contaminated with humic acid. Subsequently, a pump is employed to draw the polluted water through the ceramic membrane module for filtration, with the filtrated permeate water collected in another 5 L product tank (Fig. 1b). Backwashing is carried out using pure water and air between operation cycles to recover membrane performance and improve efficiency.

Fig. 1 CERAFILTEC lab-scale ceramic membrane filtration test unit showing a photograph and b schematic drawing of the process
The unit can replicate genuine filtration processes such as filtration, backwash on-air, backwash submerged, and air-scouring during filtration or backwash. It is capable of continuous and automatic operation, with adjustable settings, facilitated by a control system developed using Siemens LOGO with TDE software, ensuring precise and effective operation. The control unit records changes in transmembrane pressure (TMP) over time, and the volumetric flow rate can be recorded and adjusted using the control panel by altering the pump frequency. The operating conditions and filtration specifications for this unit are outlined in Table S2.
All membranes underwent testing to evaluate the influence of different composition ratios during ceramic membrane preparation and varying firing temperatures on membrane performance. The assessment involved measuring permeate flux through a pure water permeability test, followed by evaluating flux and humic acid separation efficiency at a consistent concentration of 50 mg L−1. The experimental setup operated under a maximum feeding filtration pressure of − 0.6 bar.
The water flux (Jw) in this system can be calculated using the following equation (Zhang et al. 2020):
Jw, L∕m2 ⋅ hr = Vw∕(A×t) (1)
where Vw is the volume of the filtrated water permeate (m3), A is the effective area of the membrane (m2), and t is the permeation time (h).
Additionally, humic acid separation (HR) was conducted in triplicates for each membrane, and the average result was calculated using the following equation (Zhang et al. 2020):
HR, %= Cf−Cp ∕Cf×100 (2)
where Cf and Cp are the concentrations (mg/L) of feed bulk and permeate solutions, respectively. The concentration of humic acid in both the raw water and the permeate was measured by UV absorbance at 254 nm using a Hach DR6000 UV–VIS Spectrophotometer.
Modification of selected ceramic membrane samples
After evaluating the physical and mechanical properties of twenty fired ceramic membrane samples, it was determined that only one membrane, M1-1000℃, produced from 100% ceramic sludge waste and sintered at 1000℃, exhibited the highest efficiency in separating humic acid and permeate flux. To enhance its performance, the M1-1000℃ membrane underwent modifications utilizing functionalized multi-walled carbon nanotubes (f-MWCNTs). The obtained p-MWCNTs were functionalized as previously ascribed (Rashed et al. 2020; Wang et al. 2015), and as illustrated in the SI. Prior to the modification process, the membrane underwent a cleaning procedure involving immersion in ethanol using a water bath sonicator for 15 min. Subsequently, powdered f-MWCNTs were weighed and suspended in 50 mL of ethanol to produce a 0.1 wt. % solution through sonication for 30 min (Ajmani et al. 2012). The f-MWCNTs were then applied to the membrane surface through wet impregnation and drop-casting methods. In the wet Impregnation process, the M1-1000℃ membrane was immersed in an aqueous solution of f-MWCNTs in an ultrasonic bath for 30 min to ensure uniform distribution of f-MWCNTs. It was then dried at 50℃ for 2 h to remove excess liquid (Soroush et al. 2015). For drop casting, the prepared f-MWCNT solution was carefully deposited onto the membrane surface using a micropipette. The membrane was then dried at 50℃ for 24 h and subsequently exposed to open air for 2 days to remove excess solvent and facilitate adhesion of f-MWCNTs (Gao et al. 2014). Approximately 9.6 mg of f-MWCNTs were quantified to be deposited on the membrane surface using this method.
Following these modifications, the modified membrane (M1-1000℃) was ready for use. The modified membranes underwent testing using the lab-scale ceramic membrane filtration test unit from CERAFILTEC (Fig. 2). The effect of synthetic humic acid solution concentrations ranged from 10 to 100 mg L−1 on membrane performance was assessed by determining the permeate flux and humic acid separation efficiency using specific Eqs. 1 and 2. Further characterization such as chemical stability and corrosion testing (Rawat and Bulasara 2018) was performed as ascribed in SI.
Fig. 2 Depicts the XRD patterns of a roller kiln and b ceramic sludge wastes
Results and discussion
Characterization of raw materials
XRF analysis
The chemical analysis of two ceramic waste samples is detailed in Table 2. The roller kiln waste sample is primarily composed of alumina, with silica as a secondary component. This composition is attributed to the utilization of high alumina rollers in the kiln, resulting in almost negligible loss of ignition (Amin et al. 2016). In contrast, the ceramic sludge waste sample primarily consists of silica, with alumina as the next most abundant component. Its chemical composition is similar to that of brick clay, but with a higher alumina content due to its silica composition (Ramadan et al. 2008). The observed loss on ignition value, indicating the presence of limestone and organic matter, is within a reasonable range (Roushdy 2019).
Table 2 Chemical compositions of ceramic sludge and roller kiln wastes as determined through XRF analysis

XRD-analysis
The X-ray diffraction (XRD) analysis reveals that the roller kiln waste predominantly consists of alumina, with mullite (PDF00-015-0776) and corundum (PDF01-073-5928) as the primary minerals. Additionally, minor quantities of silica, represented by silicon (PDF00-027-1402) and cristobalite (PDF00-071-0087), were identified, as depicted in Fig. 2a. In contrast, the ceramic sludge waste is primarily composed of silica, with quartz (PDF 00-005-0490) as the dominant phase. Furthermore, the presence of kaolinite (PDF 00-014-0164), albite (PDF 00-009-0466), and calcite (PDF 00-005-0586) phases was observed, as illustrated in Fig. 2b. As anticipated, the ceramic sludge encompasses a mixture of all the components present in the raw mixtures utilized for the production of wall and floor tiles.
TG/DTG-analysis
The thermal analysis (TGA and DTG) obtained by heating the powder at 10℃/min in N2 for the roller kiln waste is presented in Fig. 3a. An endothermic peak is observed in the 40–135℃ range, indicating minor atmospheric moisture loss. No other significant endothermic peaks or weight loss are observed for the alumina powder waste. The apparent weight increase on the TGA trace is likely due to a sloping baseline and can be disregarded. The negligible change in weight of the specimen up to 1000℃ is expected given the inert nature of its constituents (mullite + cristobalite) (Ahmed et al. 2014).

Fig. 3 Thermal analysis (TG and DTG curves) of a roller kiln and b ceramic tile sludge wastes
The combined TGA—DTG chart for ceramic sludge waste is depicted in Fig. 3b, revealing three zones of anomalies. The first zone exhibits an endothermic peak between 70℃ and 150℃, corresponding to the loss of water contained in the waste sample. The second zone, with an endothermic peak between 350℃ and 610℃, is attributed to the lattice water of clay due to the dehydroxylation of kaolinite to form metakaolinite, which has a poorly organized structure (Wahyuni et al. 2018). The third zone, with an endothermic peak between 615℃ and 710℃, arises from the decomposition of the calcite mineral (Buregyeya et al. 2018).
Particle size distribution (PSD)
Figure S3 displays the cumulative and differential curves for particle size analysis of roller kiln and ceramic sludge wastes. The vertical Y-axis represents the fraction passing through each screen aperture, while the horizontal X-axis shows the particle size range in micrometers (μm). Figure S3a indicates that the ground alumina in roller kiln waste is very fine, with particle diameters ranging from 0.598 µm to 87.05 µm and a median particle size (D50) of 8.282 µm. Similarly, Fig. S3b reveals that the milled silica in ceramic tile sludge waste is also fine, with particle diameters spanning from 0.610 µm to 77.00 µm and a D50 of 7.627 µm.
Powder density
The density of the roller kiln and ceramic sludge waste was determined using the density bottle method. The procedure was repeated five times to ensure accuracy, resulting in mean true densities of 3.157 g/cm3 for roller kiln waste and 2.554 g/cm3 for ceramic sludge waste, respectively. Additionally, the bulk densities were measured at 1.188 g/cm3 for roller kiln waste and 0.607 g/cm3 for sludge waste.
Characterization of ceramic membranes
Studying the physical and mechanical properties
Total shrinkage
As the firing temperature increases, both linear and volumetric shrinkage of ceramic membranes increases due to enhanced sintering, promoting particle bonding and densification. Membranes with higher roller kiln waste content (M4–M5) exhibit less shrinkage compared to those with higher ceramic sludge waste (M1–M2), as depicted in Fig. S4a and b. This difference arises from the lower or zero weight loss observed in high alumina waste samples, indicating prior pre-firing in ceramic tile processes, which reduces weight loss and minimizes shrinkage in the current sintering process. Conversely, samples with higher silica waste content exhibit partially higher shrinkage due to chemical and physical reactions during firing, leading to structural changes at the chemical and physical levels. Ceramic tiles contain a significant amount of feldspar, serving as the source of liquid phase sintering and contributing to linear and volume firing shrinkage [8]. Ceramic sludge waste also contains feldspar (albite), leading to increased firing shrinkage, while the addition of high alumina refractory waste limits or suppresses liquid phase formation, resulting in reduced firing shrinkage.
Loss on ignition (LOI), water absorption (A), and saturation coefficient (SC)
Figure 4a demonstrates that with increasing firing temperature, LOI tends to rise due to enhanced chemical reactions and moisture absorption rather than removal. However, the rate of increase may vary depending on the blend ratio. Higher ratios of roller kiln waste (M4–M5) may exhibit lower LOI due to minimal chemical reactions and the absence of decomposable material in the waste. Conversely, higher ratios of ceramic sludge waste (M1–M2) may lead to higher LOI values due to increased chemical reactions, such as limestone, kaolinite, and organic matter.

Fig. 4 Effect of firing temperature on both: a Loss on ignition, b cold water absorption, c boiling water absorption, and d saturation coefficient, of all membranes, each with varying composition of roller kiln and ceramic sludge wastes
The analysis of membrane-water interaction and thermal stress resilience, as depicted in Fig. 4b–d, reveals a correlation between water absorption behavior and firing shrinkage trends. Increased percentages of roller kiln waste correspond to decreased water absorption, while higher ceramic sludge waste percentages lead to increased absorption. This reflects the impact of ceramic sludge waste on firing shrinkage, where its addition reduces porosity, thereby decreasing water absorption rates. Conversely, the integration of roller kiln waste reduces firing shrinkage, possibly by inhibiting liquid phase formation during firing, thus increasing porosity and water absorption values.
These dynamics are crucial for evaluating how the membrane interacts with water and withstands thermal stress. Membranes with higher alumina waste ratios (M5), when exposed to various firing temperatures, particularly at 1000–1300℃, display notably high rates of cold and boiling water absorption: 28.45% and 24.82% for cold water, and 29.03% and 25.43% for boiling water, respectively. This suggests an increase in porosity and, to some extent, a reduction in densification, although the reduction is slight as temperature rises. Consequently, there is a higher saturation coefficient, indicating decreased resistance to freeze–thaw cycles. In contrast, membranes with higher silica waste concentration (M1) at 1200℃ exhibit the lowest water absorption rates of 0.11% for cold water and 0.3% for boiling water, indicating decreased porosity and enhanced glassification. Consequently, they display lower saturation coefficients, indicative of stronger resistance to freeze–thaw damage. At 900℃, an increase in water saturation of 17.96% for cold water and 18.76% for boiling water, alongside rising porosity, affects structural integrity and water interaction, resulting in higher absorption rates.
Bulk density and porosity
In Fig. 5a, the bulk density measurements indicate that increasing the proportion of roller kiln waste results in lower bulk densities (1.73–1.77 g/cm3) in the ceramic membranes (M5) at temperatures ranging from 1000℃ to 1300℃. This decrease in density is attributed to the alumina-rich nature of roller kiln waste, known for its inert properties, which contributes to less dense structures compared to those containing ceramic sludge waste. Additionally, the trend of increasing bulk density to 1.96 and 2.09 g/cm3 at higher sintering temperatures of 1200℃ corresponds to the presence of higher sludge waste content (M1 and M2, respectively). This suggests enhanced particle bonding and densification at elevated temperatures during the ceramic membrane sintering process.

Fig. 5 Effect of firing temperature on both: a bulk density, b apparent, c closed, and d total porosity of all membranes, each with varying compositions of roller kiln and ceramic sludge wastes
In Fig. 5b–d, as firing temperatures rise to 1200 and 1300℃, both the apparent and total porosity decrease. For membranes with higher silica (M1) and alumina (M5) content, the apparent porosity falls to 2.63%–42.02%, and the total porosity drops to 25.3%–45.97%, respectively. This reduction occurs because open pores convert into closed pores during sintering, which promotes denser structures.
Moreover, membranes made with a higher proportion of roller kiln waste (M5) show even lower closed porosity levels (1.22%–3.95%) at these temperatures, reflecting reduced densification. Conversely, those with a higher content of ceramic sludge waste (M1) exhibit greater closed porosity (22.67%) at 1200℃, indicating higher densification and vitrification as proved in Fig. 5c. These observations demonstrate that the type of waste used and the firing temperatures significantly impact the density and porosity of the membranes. Roller kiln waste, which is rich in alumina, tends to create less dense structures than silica-rich ceramic sludge waste. Furthermore, the increasing firing temperatures enhance particle bonding and densification, collectively contributing to the overall structural compactness of the membranes.
Compressive strength
The compressive strength of the prepared ceramic membranes was measured and evaluated as shown in Fig. S5.
Membrane performance test
Pure water permeability test
In Fig. 6, the pure water permeability as a function of membrane water flux (L/m2 h) of the prepared ceramic membranes, characterized by varying compositions and sintering temperatures, demonstrates significant variability. At 900℃, membranes M1 and M2, rich in silica waste, exhibit high flux due to pore expansion, with corresponding low pressures of around 26 mbar for M1 and slightly higher for M2, indicating minimal resistance to flow. However, as the sintering temperature increases to 1000℃ and 1100℃, the flux for these membranes decreases, and pressure rises to approximately 50.333 mbar, reflecting increased filtration resistance due to membrane densification.

Fig. 6 Impact of firing temperature on all membranes’ flux, each with varying composition of roller kiln and ceramic sludge wastes
Conversely, membranes M3, M4, and M5, with varied mixture ratios, show different flux behaviors across temperatures. M5, at 1000℃, outperforms M3 and M4 with higher flux and lower pressure (8.67 mbar), suggesting better permeability. At 1100℃ and 1200℃, M3 and M4 exhibit reduced flux and slightly increased pressure compared to M5, which maintains lower pressure, indicative of its higher permeability due to the presence of alumina waste. At the highest temperatures of 1200℃ and 1300℃, all membranes undergo a sharp decrease in flux and an increase in pressure. This is attributed to vitrification and the transformation of open pores into closed pores, which restricts fluid flow by creating a glassy phase and reducing open pore volume, thereby decreasing membrane permeability.
Humic acid rejection
The effects of firing temperature and composition variability on humic acid filtration and permeate flux at a fixed concentration of 50 mg L−1 was performed. Figure 7a and b illustrates the performance of ceramic membranes, M1 to M5, in humic acid separation, highlighting the intricate balance between flux, rejection ratio, and operational pressure across varying temperatures. For M1, at 900℃, a flux of 508.74 L/m2 h is observed with a humic acid rejection ratio of 13% at a pressure of 112 mbar. Increasing the temperature to 1200℃, the flux sharply decreases to 22.97 L/m2 h, while the rejection ratio spikes to 89%, albeit at a significantly higher pressure of 319 mbar. This suggests that elevated temperatures improve M1's rejection towards humic acid but constrict water flux, possibly due to the formation of a glassy phase that adversely impacts membrane porosity and water absorption, as evidenced in Figs. 5, 6, and 7, where the lowest water absorption correlates with vitrification properties. Similarly, M2 at 900℃ shows a flux of 507.85 L/m2 h with a 13% rejection ratio and a lower pressure of 65 mbar. However, at 1200℃, the flux diminishes to 31.22 L/m2 h, and the rejection ratio increases to 47%, with pressure rising to 210 mbar, indicating that the roller kiln component moderately enhances the membrane's filtration performance and thermal stability.

Fig. 7 Influence of firing temperature on a humic acid flux and b separation efficiency in membranes with different compositions of roller kiln and ceramic sludge wastes, at a constant concentration of 50 mg L−1
Remarkably, at 1000℃, M3 exhibits a higher flux of 540.63 L/m2 h compared to M1 and M2, with a slightly lower rejection ratio of 11% and a reduced pressure of 61 mbar. This pattern of high flux with a gradual increase in rejection continues until 1300℃, where a notable decrease to 17.86 L/m2 h in flux and a surge in rejection to 71% are observed, signaling a significant change in membrane characteristics due to extreme temperatures, including densification and pore closure. M4 showcases superior permeability at 1000 °C with the highest flux among the membranes at 556.12 L/m2 h, an 11% rejection ratio, and the lowest pressure of 56 mbar. Even at 1300℃, M4 maintains a relatively high flux of 460.96 L/m2 h with a 17% rejection ratio and a slightly elevated pressure of 96 mbar, underscoring the significant role of alumina waste in preserving membrane permeability at elevated temperatures.
M5, starting at 573.60 L/m2 h with the lowest rejection of 6% at 1000℃, demonstrates unparalleled flux capabilities, maintaining high flux up to 1300℃ at 549.56 L/m2 h and improving rejection to 7%. The consistently low pressure, from 53 to 84 mbar, accentuates M5's exceptional ability to sustain high permeability and minimal humic acid rejection across a broad temperature spectrum. However, this membrane is too porous and brittle and is of no practical value.
Modified ceramic membranes
From previously concluded results, the optimal membrane performance based on humic acid separations and permeate flux was selected for surface modification toward enhancing the humic acid rejection with suitable flux. Based on the evaluation of the twenty membranes in Fig. 7a and b, M1 and M2 at 1200℃, and M3 at 1300℃ demonstrated comparable and high humic acid separation efficiencies (89%, 47%, and 71%, respectively). However, their low permeate flux rates (22.97, 31.22, and 17.86 L/m2 h) prompted a focus on M1 at 1200℃ for its exceptional separation performance. Despite this, M1 at 1000℃ was ultimately chosen for further assessment due to its excellent balance of separation efficiency (19%) and high flux (486.07 L/m2 h) compared to the other membranes. This membrane is slated for surface modification with f-MWCNTs to enhance its separation efficiency further, following careful consideration of performance testing, physical and mechanical properties, and optimal fabrication conditions of all ceramic membranes.
Performance evaluation of the modified ceramic membrane
The findings from the wastewater treatment test using the modified M1-1000℃, as depicted in Fig. 8a–d, indicate a strong correlation between treatment efficiency and feed concentration. As the humic acid (HA) concentration in the feed wastewater increases from 10 to 100 ppm, there’s a corresponding rise in the HA concentration in the permeate water, escalating from 1.08 to 7.59 ppm due to the higher concentration in the feed. Consequently, the rate of HA separation increases from 89.17 to 92.61%. However, this rise in separation efficiency is accompanied by a decrease in permeate flux, dropping from 332.49 to 128.46 L/m2 h due to the concentration polarization phenomenon. Notably, there's a significant increase in transmembrane pressure from 180 to 210 mbar, indicating a greater shear force that reduces the membrane concentration polarization layer. This reduction positively impacts both membrane transport properties and separation effectiveness.

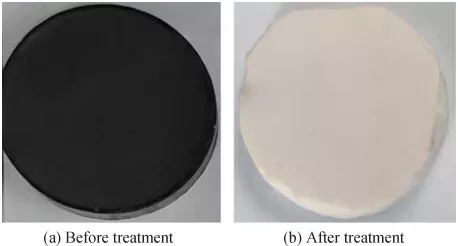
Fig. 8 The impact of varying concentrations of humic acid solutions on a the separation efficiency and flux, b the separation efficiency and transmembrane pressure, c photographs of 100 ppm humic acid solution before and after treatment using the modified M1-1000℃ membrane, and d photograph of the modified ceramic membrane (M1) at 1000℃ using f-MWCNT
Characterization of the modified ceramic membrane
The XRD patterns of the raw high silica waste, fired membrane (M1) at different temperatures (i.e., 1000 and 1200℃), and modified membrane (M1) at 1000 °C using f-MWCNTs indicated the presence of different mineralogical compositions as depicted in Fig. 9a. The characteristic peaks observed at 2θ values of 12.36°, 24.69°, and 35.05° were attributed to the presence of the kaolinite mineral (Tironi et al. 2012). Additionally, the diffraction peaks detected at 20.84° and 26.66º were indicative of the existence of quartz mineral (Zuo et al. 2016).

Fig. 9 a XRD-patterns and b FTIR-spectroscopy of raw high silica waste, fired membrane (M1) at different temperatures (i.e., 1000 and 1200℃), and modified membrane (M1) at 1000℃ using f-MWCNT
Furthermore, the minerals albite and calcite were also observed through the identification of diffraction patterns located at 24.28° and 27.97° (Sánchez-Soto et al. 2021), in addition to 29.43°, 36.59°, and 39.47° (El-Mahllawy et al. 2018), respectively. The Sintering of the membrane (M1) sample at 1000℃ resulted in the deformation of calcite and kaolinite minerals, as provided by the disappearance of their related peaks, along with enhancing the intensities of the albite characteristic peaks.
Rising the firing temperature up to 1200℃ caused the appearance of a new mullite phase, as distinguished at 30.51°, 39.44°, 42.37°, and 47.28° (Gong et al. 2014). Adding f-MWCNTs to the fired membrane sample (M1) at 1000℃ resulted in the appearance of new diffraction peaks positioned at 26.82° and 45.97° (Cao et al. 2001), which confirmed the physical adhesion between the fired membrane sample and functionalized-MWCNTs.
Figure 9b shows the FTIR spectrum of raw high silica waste and fired membrane (M1) samples at different temperatures (i.e., 1000 and 1200 °C), besides the modified membrane (M1) at 1000℃ using f-MWCNT. As for raw ceramic sludge waste, the observed band detected at 3665 cm−1 could be related to the hydroxyl stretching within kaolinite internal structure (Zuo et al. 2018). The 700–1200 cm−1 range exhibits Si–O and Al–O stretching modes, while the 150–600 cm−1 range is dominated by Si–O and Al–O bending modes of the kaolinite mineral (Plevova et al. 2020). The sintering of the membrane (M1) sample at 1000℃ results in a reduction of the intensity of the chemical water peak and a slight shift to lower values (from 3665 to 3643 cm−1). Furthermore, distinct changes in the positions of the Si–O and Al–O stretching and bending modes are observed, accompanied by the appearance of new peaks at 459 and 1728 cm−1. These changes may be attributed to the deformation of kaolinite minerals and the initial crystallization of new phases. Elevating the firing temperature at 1200℃ leads to enhancing the intensity of the pre-existing absorption bands and the formation of new peaks detected at 2983, 1582 cm−1. This could be linked to the nucleation/crystallization process of mullite mineral as proved by XRD-data, see Fig. 9. Additionally, the incorporation of f-MWCNTs to the fired membrane (M1) at 1000℃ resulted in the detection of a new peak located at 1722 cm−1 of the C=O stretching vibration (Hassani et al. 2022).
The chemical stability of the modified membrane M1-1000℃ compared to the pristine ones was evaluated and presented as shown in Fig. S6.
The micrographs obtained from SEM-analysis at various magnifications, along with the EDX results of fired ceramic membranes at different temperatures (specifically, 1000 and 1200℃), as well as fired membrane sample modified with f-MWCNTs at 1000℃, are presented in Fig. 10. The surfaces and cross-sectional images and EDX-data of the sintered membrane at 1000℃ (Fig. 10a and b) depict a porous structure and a moderately sorted distribution of the fired grains, primarily composed of silica and alumina. This composition indicates effective water contaminant treatment efficiency, as evidenced in the measured porosity, along with high levels of porosity and water absorption observed in the analysis of the physical properties of the base membranes. The elevation of temperature to 1200℃ leads to the disruption of the porous structure of the fired membrane sample due to the partial melting of the aluminosilicate phases, resulting in the formation of a solid glassy phase (liquid phase) (Abdallah et al. 2018), as evidenced in the surfaces and cross-sectional micrographs (Fig. 10c and d). Hence, the optimal firing temperature for preserving the porous structure is 1000℃. To improve the separation efficiency of the synthesized membrane, f-MWCNTs were integrated to reduce the porous structure size to the nanoscale. The introduction of f-MWCNTs to the membrane sample fired at 1000℃ resulted in the emergence of well-distributed dendritic/forked shapes on the external surface of the ceramic membrane, as illustrated in the surface image in Fig. 10e. This observation substantiates the homogeneous spreading of the f-MWCNTs on the membrane sample surface, which was further confirmed by the EDX-outcomes, indicating the presence of F-MWCNTs on its surface. Moreover, the cross-sectional image depicted in Fig. 10f, and its EDX analysis demonstrate the presence of f-MWCNTs within the internal structure of the f-MWCNTs-modified membrane sample. Therefore, the presence of f-MWCNTs on the surface and within the internal structure of the fired membrane sample elucidates the enhanced separation efficiency of this sample as proved in Fig. 8 when compared to the fired membrane sample at 1000℃.

Fig. 10 SEM–EDX analyses of fired ceramic membranes at different temperatures a and b at 1000℃, c and d, at 1200℃, and e and f fired membrane at 1000℃ modified with f-MWCNTs
The pore size distribution of the modified M1-1000℃, compared to M1-1000℃, and M1-1200℃ showed an agreement with the obtained results from the surafce microstructure analysis by SEM, as shown in Fig. S7.
Conclusion
Affordable eco-effective ceramic nano-filtration and ultra-filtration membranes were developed for the treatment of humic acid-contaminated water, utilizing silica-rich ceramic sludge and alumina-rich roller kiln waste as raw materials. The membranes were fabricated using different compositions and firing temperatures (900 to 1300℃). Exhaustive characterization of the ceramic powders was performed using various analytical techniques, including XRF, XRD, TGA-DTG, and particle size distribution.
Performance assessment revealed substantial impacts of composition ratio and firing temperature on separation efficiency and flux. Among the tested compositions, membranes fired at 1000℃ using solely ceramic sludge waste exhibited an optimal balance, achieving 19% separation efficiency and 486.07 L/m2 h flux at 50 ppm humic acid solution concentration. These membranes demonstrated adequate mechanical strength to withstand water pressure. Additionally, the surface modification of the optimal membrane sample (M1-1000℃) with f-MWCNTs significantly enhanced its separation efficiency to 92.61% and permeate flux to 128.46 L/m2 h at 100 mg L−1 humic acid solution concentration and 210 mbar transmembrane pressure. SEM analysis confirmed numerous small nano-sized pores on the membrane's surface and internal structure, while XRD and FTIR analyses identified the presence of quartz, albite, carbon, Si–O, Al–O, and C=O functional groups in the modified membrane. Ultimately, this study illustrates the potential of utilizing ceramic waste materials for effective membrane technologies in water treatment, offering promising solutions for addressing humic acid pollution challenges.
Future research could expand upon exploring further applications of the developed ceramic membrane in treating industrial wastewater contaminated with pollutants other than humic acid. Additionally, there is potential to enhance the membrane’s properties and performance through conducting more experiments and practical applications, which would contribute to boosting its efficiency and effectiveness in industrial and environmental applications.
Reference: Omitted
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.