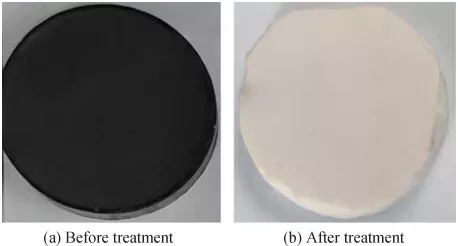
We fabricated highly aligned porous silicon carbide (SiC) ceramics with well-defined pore structures by freezing a polycarbosilane (PCS)/camphene solution. In this method, the solution prepared at 60℃ was cast into a mold at temperatures ranging from 20° to ~196℃, which resulted in a bicontinuous structure, in which each phase (camphene or PCS) was interconnected in a regular pattern. After the removal of the frozen camphene network, the samples showed highly porous structures, in which long straight and short elongated pore channels were formed parallel and normal to the direction of freezing, respectively. Thereafter, porous SiC ceramics were produced by the pyrolysis of the porous PCS objects at 1400℃ for 1 h in a flowing Ar atmosphere, while preserving their mother pore structures having aligned pore channels.
I. Introduction
POROUS silicon carbide (SiC) ceramic is of technological importance for a wide range of applications, such as catalyst supports1 and filters for hot gas or molten metal,2 due to its good thermal shock resistance, high mechanical properties, and excellent chemical stabilities at elevated temperatures.3,4 The mechanical properties and performances of porous ceramics are strongly dependent on not only the intrinsic properties of the material used as the framework but also their pore structures, such as their pore size, distribution, interconnection between pores, and pore orientation.5 Thus, the preferred manufacturing method should be able to tightly control the pore structure of the ceramics in order to optimize their properties.
Nowadays, a number of manufacturing techniques are available for the production of porous SiC ceramics using either ceramic powders or preceramic precursors. The former methods, including the polymer replication method6,7 and the oxidation bonding technique,8 normally require relatively high temperatures to sinter the SiC particles, accordingly limiting their practical application. Freeze casting using either water9,10 or camphene11–13 as the freezing vehicle is also currently accessible, wherein pore channels are formed as the replica of frozen vehicle dendrites. On the other hand, the latter methods exploit the fact that the porous preceramic foam can be converted to the SiC ceramics by heat treatment (i.e., pyrolysis) at lower temperatures.14,15 As a preceramic precursor, polycarbosilane (PCS) is a representative polymer extensively used for the fabrication of SiC fibers.16,17 However, none of the above-mentioned methods is capable of tightly controlling the pore structures, for example, producing aligned pore channels.
Recently, biomorphic porous ceramics produced using natural woods as sacrificial templates have attracted increasing interest,18–20 as they can mimic the cellular structure of the wood template that has optimal properties from the viewpoint of the mechanical property/density relationship and fluid transport.21 These materials would find very useful applications in diverse fields, especially those in which high mechanical properties are required.22 However, this approach suffers from the problem of there being a limited selection of available natural templates.
Herein, we demonstrate a simple, innovative method of fabricating highly aligned porous SiC ceramics, whose structures resemble the cellular structure of natural materials, such as woods and nacres, by freezing a PCS/camphene solution. This method exploits the fact that the warm solution prepared at 60℃ undergoes thermally-induced phase separation (TIPS) during freezing, which has been extensively used for the preparation of highly porous polymer foams.23–25 This phase separation would allow the formation of a well-defined pore morphology. The porous PCS preceramic produced after the removal of the frozen camphene via freeze drying can be readily converted into the porous SiC ceramic via simple pyrolysis at 1400℃ for 1 h in a flowing Ar atmosphere, while preserving its aligned porous structure. The processability and the control over the pore structures are discussed.
II. Experimental Procedure
(1) Starting Materials
A commercially available PCS (Type A, Nippon Carbon Co. Ltd., Yokohama, Japan) was used as the preceramic precursor for the fabrication of the SiC ceramic. The as-received solid PCS was crushed into fine granules, in order to promote its dissolution behavior in the solvent. A commercial camphene (C10H16; Alfa Aesar/Avocado Organics, Ward Hill, MA) was used without further purification as the freezing vehicle, as it can freeze at moderate temperatures due to its melting point of 44°–48℃ and easily sublime even at room temperature.18,19
(2) Freeze Casting
Firstly, PCS/camphene solutions with various PCS concentrations, ranging from 5 to 20 wt% in relation to the camphene, were prepared by dissolving solid PCS granules in the molten camphene at 60℃ for 3 h using a magnetic stirrer. This simple process allowed the formation of a homogenous and clear solution without difficulty. The prepared warm solutions were then poured into polyethylene molds with an inner diameter of 12.5 mm pre-heated to 60℃. Thereafter, cool ethanol or liquid nitrogen was supplied to induce directional freezing from the mold wall to the center of the cast body, as shown in Fig. 1(A). In order to control the pore structure, the freezing temperature (i.e., the temperature of the cool bath) was adjusted, in the range from 20° to -196℃.

Fig. 1. Schematic illustrations showing (A) the experimental setup used for the freeze casting of the polycarbosilane/camphene solution at a controlled temperature and (B) the phase separation during freezing, where the camphene grows dendritically parallel to the freezing direction, accordingly resulting in a regular bicontinuous structure.
Immediately after the freeze casting, solidification took place starting from the mold wall, which was much cooler than the cast body. Under these conditions, the heat of the warm solution dissipated through the solid, causing the camphene to grow dendritically toward the center of the cast body, accordingly resulting in the formation of a bicontinuous structure, in which each phase (camphene or PCS) is interconnected in a regular pattern (Fig. 1(B)). Typically, <30 min was required for the complete solidification of the cast body, i.e., the freezing of the solutions.
(3) Camphene Sublimation and Heat Treatment
Before demolding, the frozen bodies were placed in a cool atmosphere at around -68℃ to enhance their green strengths. The green bodies were removed from the molds, followed by
freeze drying for 12 h to completely remove the frozen camphene without disintegrating the porous structures of the samples. It should be noted that the porous PCS did not show any noticeable morphological or microstructural changes, as well as negligible linear shrinkage, after freeze drying. The prepared samples were then transferred to an alumina crucible and heat treated, first at 200℃ for 3 h in air at a heating rate of 0.3℃/min to cross-link the PCS via oxidation curing, and then by pyrolysis at 1400℃ for 1 h in a flowing Ar atmosphere to convert the PCS preceramic to SiC ceramic, which caused the samples to shrink to a certain extent.
(4) Characterizations
The melting and crystallization points of the PCS/camphene solutions were measured by differential scanning calorimetry (DSC; DSC Q 1000, TA Instruments, Crawley, U.K.). For
the purpose of comparison, pure camphene was also examined. The solution was quenched to solidification in cold water. A small piece was then sliced off and placed in a volatile-sample pan for the DSC measurements. The sample was first heated to 60℃ at a heating rate of 2℃/min, held at this temperature for 10 min, and then cooled at the same rate.
The viscosities of the PCS/camphene solutions were measured on a rheometer (AR2000, TA Instruments, New Castle, DE) with a cone-and-plate measurement geometry. The temperature used for the measurements was controlled by the indirect heating of the lower plate, and set at 60℃. A solvent trap was used to minimize the evaporation of the camphene.
The TIPS behavior of the PCS/camphene solution was examined using an optical microscope (Leica DM LP; Leica Microsystems, Wetzlar, Germany) equipped with a hot stage. The sample was prepared by dropping the solution with a PCS concentration of 10 wt% onto a slide glass and cover glass. During freezing, the changes in the microstructure were directly observed using a polarized optical microscope.
The chemical structures of the samples were analyzed with Fourier transform-infrared spectroscopy (FT-IR; Nicolet Magna 550 series II, Midac, CA) in the Mid-IR spectrum range using a KBr pellet within the frequency range of 400–4000 cm-1 at a scan rate of 5 (cm·sec)-1 . In addition, the crystalline structures of the samples were analyzed by X-ray diffraction (XRD, M18XHF-SRA, MAC Science, Yokohama, Japan) with CuKa radiation within the 2y range of 20°–80°.
The fabricated samples were characterized by evaluating the development of the pore structure, such as the pore orientation, pore size, pore shape, and degree of interconnection, as well as the morphologies of the PCS and SiC walls using optical microscopy (PMG3, Olympus, Tokyo, Japan) and scanning electron microscopy (SEM, JSM-6330, JEOL Techniques, Tokyo, Japan). The pore structure was directly observed from the fractured samples. The pore size, which is defined by the secondary dendrite arm spacing (SDAS), were roughly measured using a linear intercept method.
III. Results and Discussion
(1) Material Selection for Freezing
We used the freeze casting of a PCS/camphene solution for the production of highly porous SiC ceramics with well-defined pore structures, where the solution temperature is lowered to induce the phase separation of the homogenous polymer solution, through a process known as TIPS.23 Pores are readily formed by removing the frozen solvent. This TIPS method has been widely used for the preparation of highly porous polymer foams24,25 and inorganic nanoparticle embedded polymer foams.26
In order to take full advantage of the TIPS method, it is crucial to select a suitable polymer and organic solvent. As the polymer, PCS is selected as it is a representative preceramic precursor for the production of SiC fibers16,17 and is versatile and has a wide range of applications; for instance, it can also be used as the basic component for producing photocatalytic TiO2-covered silica fibers.27 Furthermore, PCS is known as a valuable source for the growth of SiC whiskers (or wires), when treated under appropriate conditions,28,29 which would make it possible to achieve unique pore channels. Many kinds of organic solvents, such as tetrahydrofuran,30 xylene,31 and N-hexane,32 can completely dissolve a PCS; however, these solvents normally require relatively low temperatures for freezing (-108°, -25° to 13°, -95℃ for tetrahydrofuran, xylene, and N-hexane, respectively) and are rather detrimental to health. Thus, we examined several organic solvents and empirically found that camphene could dissolve PCS preceramic well by a simple magnetic stirring process. Camphene (C10H16) is a cyclic hydrocarbon and crystalline plastic solid at room temperature, due to its low melting point of 44–48℃, which allows it to be frozen even at room temperature. In addition, camphene is derived from a natural terpenoid; thus, it is environmentally friendly. These properties suggest that camphene is one of the most promising solvents that can be used to induce the TIPS of a PCS solution.
(2) Melting and Freezing Behavior
It was observed that, regardless of the PCS contents, ranging from 0 to 20 wt%, all of the prepared solutions showed very low viscosities, as summarized in Table I. The sample even with the highest PCS content of 20 wt% showed a viscosity as low as 3.7 mPa·s at a shear rate of 100 s-1. The melting and freezing behaviors of the PCS/camphene solutions prepared at 60℃ were characterized using DSC. The phase diagram of the PCS/camphene solution is shown in Fig. 2. As the PCS content increased from the 5 to 25 wt%, the freezing point, at which the phase separation would occur, noticeably decreased from 36.2 to 23.8℃, while the melting point decreased from 381 to 32.9℃.
Table I. Viscosities of the PCS/Camphene Solutions With Various PCS Contents, Ranging from 0 to 20 wt%, Measured at 60℃ at a Shear Rate of 100 s-1


Fig. 2. Phase diagram of the polycarbosilane/camphene solution. Tm and Tc represent the melting and crystallization temperatures, respectively.
(3) Phase Separation During Freezing
The TIPS behavior of the PCS/camphene solution during freezing was directly observed using a polarized optical microscope equipped with a hot-stage. A thin layer of the warm solution with a PCS content of 10 wt% prepared at 60℃ was placed between glass slides and cooled. After reaching the freezing point, the camphene immediately began to grow dendritically toward the upper right-hand corner, while the PCS polymer became concentrated between the dendritic arms, as shown in Fig. 3(A). It is well known that camphene can form dendrites when solidified under an appropriate temperature or solute gradient.33,34 After the freezing was completed, a unique phaseseparated structure was produced, in which aligned camphene dendrites with a well-defined morphology (oriented and uniform dendrite arms) surrounded by PCS networks were formed, as
shown in Fig. 3(B). Long straight and short elongated camphene dendrites were formed parallel and normal to the direction of the primary camphene growth, respectively.

Fig. 3. Typical optical photographs of the polycarbosilane (PCS)/camphene solution with a PCS concentration of 10 wt% during freezing, showing (A) the dendritic growth of the camphene at the early stage of freezing and (B) the regular bicontinuous structure after freezing is completed.
(4) Effect of Freezing Temperature on Pore Structure
We firstly examined the effect of the freezing temperature on the development of the pore channels by freezing the PCS/camphene solution with a PCS content of 10 wt% at controlled freezing temperatures (20°, 0°-20°, -40°, and -196℃). Camphene is usually removed via sublimation at room temperature.11–13,25 However, we found that the sublimation of the frozen sample at room temperature caused the destruction of the porous structure, presumably due to the remixing of the phase-separated solution or remelting of the frozen solution. Thus, the freezedrying technique was used to avoid this problem; it allowed the production of porous PCS objects in the form of monoliths without noticeable large defects, such as cracking or distortion.
The pore structures of the porous PCS objects prepared at various freezing temperatures were characterized using SEM. All of the fabricated samples showed similar pore morphologies, namely, regular patterns, wherein long straight and short elongated channels were formed parallel and normal to the direction of the camphene growth, respectively, while the pore channels became smaller with decreasing freezing temperature, as shown in Fig. 4(A)–(D). In addition, thinner PCS walls were observed at lower freezing temperatures.

Fig. 4. Scanning electron microscopy micrographs of the porous polycarbosilane (PCS) objects prepared at freezing temperatures of (A) 20°, (B) 0°, (C) -20°, and (D) -40℃, using a PCS concentration of 10 wt%.
During freezing, only those camphene crystals with a preferential orientation close to the direction of heat conduction can overgrow dendritically toward the center of the cast body,33 which accordingly produces long aligned pore channels. At the same time, the side arms of the camphene dendrites are also formed normal to the direction of the primary camphene dendrite, resulting in short elongated pore channels. It should be noted that the sizes of the camphene dendrites are dependent on the freezing rate, which is controlled by adjusting the freezing temperature in this study.
(5) Effect of Freezing Temperature on Pore Size
It was found that as the freezing temperature was lowered, the pore channels became narrower. To clarify this tendency, we measured short widths of the pore channels formed perpendicular to the direction of the freezing, which represented the SDAS, as shown in Fig. 5. The pore size was abruptly decreased from 23 to 11 mm by lowering the freezing temperature from 20°to 0℃. However, at the lower freezing temperatures, the reduction in the pore size was not much (from 9 to 2.7 μ on lowering the freezing temperature from -20° and -196℃). It should be noted that the sample even frozen in liquid nitrogen showed a highly porous structure, implying the effectual growth of camphene dendrites during freezing.

Fig. 5. Pore widths of the porous polycarbosilane (PCS) objects prepared with a PCS concentration of 10 wt% as a function of the freezing temperature.
It is known that the pore size would be expected to be inversely proportional to the solidification velocity by a power law relationship,35 which could be practically modified by adjusting either the cooling rate or the degree of undercooling. The measured pore sizes (λ), shown in Fig. 5, were plotted against the degree of undercooling (ΔT), as shown in Fig. 6. The data are well described by a straight line with the exponent 0.76, indicating a power law relationship between λ and ΔT:
λ=186(ΔT)-0.76 (1)

Fig. 6. Pore widths of the porous polycarbosilane (PCS) objects prepared with a PCS concentration of 10 wt% as a function of the degree of undercooling (ΔT).
Therefore, it is reasonable to suppose that a larger degree of undercooling would be expected to lead to a higher solidification velocity, consequently resulting in the formation of narrower camphene dendrites with thinner PCS walls, as shown in Fig. 7. It should be noted that this tendency would become weaker due to its power relationship between the pore size and degree of undercooling. In addition, it was observed that, as the PCS content increased from 5 to 25 wt%, the pore size did not much change, while the width of the PCS wall increased. These observations indicate that the pore size is primarily determined by the degree of undercooling.

Fig. 7. Schematic illustration showing the formations of the pore channels, depending on the freezing temperature. A lower freezing temperature would lead to a higher degree of undercooling and higher solidification rate, which, consequently, would result in the formation of narrower pore channels with thinner polycarbosilane walls.
(6) FT-IR Analyses
In order to determine whether the camphene used as the solvent altered the chemical structure of the PCS polymer or not, the chemical functional groups in the fabricated porous PCS object were characterized using FT-IR analyses. For the purpose of comparison, the as-received PCS was also examined. Both samples showed very similar absorption bands, as shown in Fig. 8, while only traces of the bands associated with camphene were visible for the porous PCS sample (Fig. 8(B)). All of the observed bands correspond well to those reported in the literature,36,37 with the major bands of Si–CH3 (1250 cm-1) and Si–H (2100 cm-1) being present along with bands due to C–H, Si–CH2–Si, and Si–OH bonds. These results indicate that camphene is a very useful organic solvent, as it allows the PCS to be dissolved without deteriorating the chemical structure
of the PCS.

Fig. 8. Fourier transform-infrared spectroscopy spectra of (A) the asreceived polycarbosilane (PCS) and (B) the fabricated porous PCS object.
(7) Curing of Porous PCS Objects
The porous PCS objects were cured by heating them at 200℃ for 1 h in air at a slow heating rate of 0.3°C/min, in order to render the PCS walls infusible during the subsequent high-temperature pyrolysis. It is well known that oxidation curing involves the cross-linking of the PCS structure with oxygen, resulting in the formation of Si–O–Si and Si–O–C bonds by the oxidation of Si–H and Si–CH3 bonds.37,38 The oxidation curing of the porous PCS sample was characterized using FT-IR analysis, as shown in Fig. 9. It was observed that an absorption bandappeared at 1710 cm-1 due to C =O stretching, along with bands due to C–O bonds and the deformation band of H2O. The observed bands are well matched with those reported in the literature,37 indicating that oxidation curing is an effective method of cross-linking the structure of the porous PCS sample
prepared by the present method.

Fig. 9. Fourier transform-infrared spectroscopy spectrum of the porous polycarbosilane object cured at 2001C for 3 h at a heating rate of 0.3°C/min in air.
(8) Conversion of PCS to SiC Ceramic
The cured porous PCS objects were then heat treated at 1400℃ for 1 h in a flowing Ar atmosphere to convert the PCS to SiC ceramic walls. A typical optical micrograph of the sample prepared at a freezing temperature of 0℃ is shown in Fig. 10. The sample showed excellent shape tolerance, while its color changed from white to black. A linear shrinkage of approximately 27% was observed. In general, when PCS preceramic is pyrolyzed, it undergoes significant weight loss, gaseous evolution, and large volume shrinkage, which often results in the formation of large pores, cracks, and distortion.39 However, the porous PCS produced in this study is comprised of thin PCS walls, thus allowing for its successful conversion to SiC without generation of any of the above-mentioned defects.

Fig. 10. Typical optical micrograph of the porous SiC ceramic after pyrolysis at 1400℃ for 1 h in a flowing Ar atmosphere (scale in ruler = 1 mm).
(9) Porous SiC Ceramics
The pore structures of the porous SiC ceramics and the densification of the SiC walls were evaluated using SEM analyses. A typical SEM micrograph of the sample produced at a freezing temperature of 0℃ is shown in Fig. 11(A) and (B). The sample showed a well-defined pore structure mimicking the mother pore structure of the porous PCS, as shown in Fig. 11(A), in which long, straight, and short elongated channels were formed parallel and normal to the freezing direction, respectively. In addition, the SiC walls formed after the pyrolysis of the PCS preceramic walls did not show any noticeable defects, such as cracking or large pores, as shown in Fig. 11(B), which should ensure that it has excellent mechanical properties.

Fig. 11. Typical scanning electron microscopy micrograph of the porous SiC ceramic after pyrolysis at 1400℃ for 1 h in a flowing Ar atmosphere showing (A) the regular pore channels and (B) the construction of the SiC walls.
(10) Crystalline Structure
The conversion of the PCS to SiC ceramics was confirmed using XRD analyses. A typical XRD pattern of the porous SiC ceramic produced at a freezing temperature of 0℃ is shown in Fig. 12. The sample showed three representative peaks for polycrystalline β-SiC, where the strong peak at 2θ = 36° corresponded to the diffraction line from the (111) lattice plane of polycrystalline β-SiC. Relatively broad peaks were observed, due to the suppressed crystallization, as is often observed in SiC ceramics produced from oxidation-cured PCS preceramic.40

Fig. 12. X-ray diffraction pattern of the porous SiC ceramic after pyrolysis at 1400℃ for 1 h in a flowing Ar atmosphere.
IV. Conclusions
Highly aligned porous SiC ceramics with well-defined pore structures were fabricated by freezing a PCS/camphene solution, whereupon the warm PCS/camphene solution prepared at 60℃ underwent TIPS separation, resulting in the formation of a regular bicontinuous structure. It was found that the camphene used as a solvent did not alter the chemical structure of the PCS preceramic, thereby allowing the production of porous SiC ceramics after removing the frozen camphene via the freeze drying and pyrolysis of the PCS preceramic at 1400℃ for 1 h in a flowing Ar atmosphere. The fabricated sample showed a regular pore structure, in which long, straight, and short elongated channels were formed parallel and normal to the freezing direction, respectively, in addition to exhibiting the crystalline structure of β-SiC. In addition, it was observed that the pore channels became narrower with decreasing freezing temperature. These results indicate that the freezing of a PCS/camphene solution is a very feasible method of producing highly porous SiC ceramics with a well-defined pore structure, which should find very useful applications in diverse fields.
References:omit
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.