Abstract: Hetero-element doped lithium orthosilicates have been considered as advanced tritium breeders due to the superior performances. In this work, Li4Si1–xTixO4 ceramics were prepared by proprietary hydrothermal process and multistage reactive sintering. The reaction mechanism of Li4Si1–xTixO4 was put forward. XRD and SEM analyses indicate that insertion of Ti leads to lattice expansion, which promotes the grain growth and changes the fracture mode. The compressive tests show that the crush load increases almost four times by increasing x from 0 to 0.2. However, the thermal conductivity and ionic conductivity are the best when x=0.05 and x=0.1, respectively. Thermal cycling stability of Li4Si1–xTixO4 pebbles was further appraised through investigating the changes of microstructure and crush load. After undergoing thermal cycling, the Li4Si1–xTixO4 still show higher crush load compared with Li4SiO4, despite Ti segregation in some samples. The x=0.05 sample exhibits excellent thermal cycling stability. In summary, proper amount of Ti doping can improve the crush load, thermal and ionic conductivity, and thermal cycling stability of Li4SiO4.
Keywords: tritium ceramic breeders; Li4Si1–xTixO4; solid solutions; crush load; conductivity; thermal cycling
1 Introduction
Tritium breeding materials are taken as one of the key functional materials for fusion reactor blanket. Neutrons generated from the reaction between deuterium (D) and tritium (T) can react with Li atom to produce tritium, thus achieving tritium self-sustaining of a D–T fusion reactor [1–3]. With preferable chemical stability, efficient tritium extraction, high temperature resistance, and no magnetohydrodynamic effect, lithium-based ceramics have long been recognized as promising tritium breeding materials [2,4–6]. However, the overall performance of conventional lithium ceramics (i.e., Li4SiO4, Li2TiO3, Li2O, LiAlO2, Li2ZrO3, etc.) is still difficult to achieve satisfactory results. In order to improve their corresponding performance, diverse advanced tritium breeding materials, such as Li2+xTiO3+y[7–9], mixed-phase ceramics represented by Li2TiO3/Li4SiO4 [10–13], oxide modified ceramics [14–16], and hetero-element doped ceramics (notably Li4+xSi1–xAlxO4 and Li2Ti1–xZrxO3) [17–20] have been prepared in recent years. Previous studies reported that Al doped lithium orthosilicate exhibited the enhanced crushing strength [17,19,20], thermal and ionic conductivity [17,20–22], and tritium release performance [18]. With respect to fusion reactor maintenance and waste disposal, it is hoped that the elements constituting the tritium breeding material have low neutron activation and short-lived radionuclides. But 26A1 has a half-life of 7.2×105 years [23].
Since tritium breeding materials should endure long periods of harsh operating conditions (high temperature, thermal gradient, irradiation by neutron and energetic particles), thermal and irradiation stability are two important parameters and deserve more attention. However, the influence of doping elements on the thermal cycling stability of tritium breeding materials has not been investigated so far. Meanwhile, fine-grained ceramics are identified as the most promising, regarding tritium release behavior and mechanical strength [24]. But with the grain refinement, surface energy of ceramic particles increases, and thus the structural and functional instability of materials may get more acute in high temperature environment. Therefore, it is significant to fabricate fine-grained lithium ceramic solid solution and investigate its thermal cycling stability.
The aim of this work is to prepare Li4Si1–xTixO4 by proprietary hydrothermal process and multistage reactive sintering (Ti has lower neutron activation than Al), and to investigate the influences of Ti doping on the microstructure, mechanical and physical properties of Li4SiO4. Furthermore, the thermal cycling stability of Li4Si1–xTixO4 pebbles is further studied. These results can cast light for future development of advanced tritium breeding materials.
2 Materials and method
Lithium hydroxide monohydrate (LiOH·H2O, purity ≥ 99%), fumed SiO2 (Hydrophilic-300, purity 99.8%), and TiO2 (30 nm, anatase, hydrophilic, purity 99.8%) were purchased from Aladdin Ltd. (Shanghai, China). All chemicals were used without further purification.
2. 1 Preparation of precursor powders
The precursor powders were hydrothermally synthesized using LiOH·H2O, fumed SiO2, and TiO2 nanopowders with a ratio of 4:(1–x):x (x=0, 0.05, 0.1, 0.2). Briefly, LiOH·H2O (0.14 mol) was thoroughly dissolved in 70 mL of deionized water under magnetic stirring. Meanwhile, fumed SiO2 (0.035(1–x) mol) was uniformly dispersed in 70 mL of ethanol. Subsequently, the above two solutions were mixed under magnetic stirring at room temperature. After that, TiO2 nanopowders (0.035x mol) were added, and continued stirring for 30 min. Then, the mixed solution was transferred into a Teflon-lined stainless-steel autoclave with a capacity of 200 mL and performed at 180 ℃ for 12 h. Finally, hydrothermal products were dried at 80 ℃ for overnight, and ground in an agate mortar to obtain precursor powders.
2. 2 Fabrication of Li4Si1–xTixO4 pebbles
The as-prepared precursor powders and deionized water were mixed in a mass ratio of 5:4 to form the slurry, and then the green spheres could be produced by dropping the slurry through a nozzle (0.7 mm in diameter) into a container of liquid nitrogen. By freezing for more than 15 min, the green spheres were salvaged and placed on filter papers, dried in air for 30 min and then in drying oven of 70 ℃ for overnight. Li4Si1–xTixO4 pebbles could be finally obtained by sintering the dried green spheres in a box-type resistance furnace. To be specific, the samples were heated to 420 ℃, dwelling for 1h, then heated to 710 ℃, dwelling for 1h, and finally sintered at 800 ℃ for 4h. The heating
rate was 5 ℃/min.
2. 3 Characterization
Thermal behavior of the precursor powders was studied by thermogravimetry and differential scanning calorimetry (TG/DSC, NETZSCH 409 PC) in air at a constant heating rate of 10 ℃/min. The phase composition and crystal structure were investigated by X-ray diffractometry (XRD-7000, Shimadzu, Japan), and the cell parameters were refined via Jade 6.5 software. The morphology and structural studies were conducted on a scanning electron microscope (SEM, Model S-4800, Hitachi, Japan) attached with an energy-dispersive X-ray spectroscopy (EDS). Grain size was measured by Nano Measure 1.2 software from SEM images. The density was measured by an electronic density balance using ethyl alcohol as the immersion medium. The crush load was tested by a universal material strength-testing machine with a 5 kN load cell and cross-head speed of 0.1 mm/min (HT-2402, Hungta). To minimize the influence caused by the pebble size, the pebbles with a diameter of 1.2–1.3 mm were used for the test. The average crush load was estimated based on the results of no less than ten pebbles. For thermal and ionic conductivity tests, the precursor powders were pressed into pellets (12.7 mm in diameter and about 1.5 mm in thickness) and sintered at the same sintering parameters. The thermal conductivity was measured by LFA 457 Micro Flash Analyzer of NETZSCH. The ionic conductivity was determined by alternating current impedance spectra (ACIS) measured using an Agilent E4980A impedance analyzer in the frequency range from 100 to 106 Hz, and analyzed by fitting the equivalent circuit model using the ZView software. For thermal cycling tests, the ceramic pebbles were heated to 800 ℃ in a box-type resistance furnace, dwelling for 4 h, then cooled down to room temperature (set the heating and cooling rate to 5 ℃/min). After each three cycles, a batch of ceramic pebbles were fetched out for microstructure characterization and compressive strength testing.
3 Results and discussion
3. 1 Phase composition of precursor powders
Figure 1 shows the XRD patterns of the precursor powders synthesized via hydrothermal process. The undoped samples consist of Li2SiO3 (JCPDS No.29-0829) and Li2CO3 (JCPDS No. 87-0728), suggesting LiOH and SiO2 tend to generate stable Li2SiO3 instead of Li4SiO4 in the hydrothermal reaction system, and the rest of Li+ changes to Li2CO3 when drying the hydrothermal products in a forced-air convection oven (2Li+2OH-+CO2→Li2CO3+H2O). The diffraction peaks of the Ti doped samples are basically the same as that of the undoped one, except the emergence of LiOH (JCPDS No. 85-0736) and Li2TiO3 (JCPDS No.03-1024) peaks. Moreover, with the increase of Ti content, the proportion of Li2CO3 decreases. It reveals there are two competitive reactions.
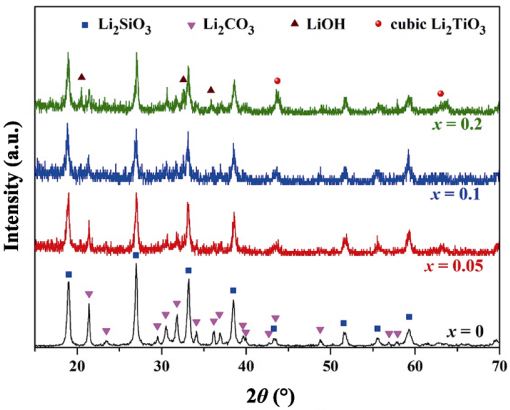
Fig. 1 XRD patterns of the precursor powders synthesized by hydrothermal method.
3. 2 TG/DSC analyses
The TG/DSC curves of precursor powders with varying Ti doping amount are illustrated in Fig. 2. The weight loss below 100 ℃, accompanied by an endothermic peak around 100 ℃, is ascribed to the removal of adsorbed water. The slight weight loss and endothermic peak around 425 ℃ can be ascribed to the reaction of residual SiO2/TiO2 with Li2CO3 [25]. Major weight loss occurs in the range of 650–750 ℃, accompanied by a sharp endothermic peak at 709 ℃, which is attributed to the formation of Li4Si1–xTixO4. The weight loss decreases with increasing Ti content due to the reduced lithium carbonate in the samples, in accordance with XRD results. Compared with the undoped sample, the endothermic peak of Ti doped samples shifts to lower temperature, indicating Ti doping can reduce the reaction temperature and raise the reaction efficiency. This is due to the lattice distortion caused by the substitution of Ti4+ for Si4+, which may reduce the activation energy [26]. No obvious weight changes are found above 800 ℃, suggesting the synthesis process is finished. Moreover, considering the weight losses around 425 and 710 ℃, multistage reactive sintering is adopted to reduce the pores and impurities (i.e., 420 ℃ for 1 h, 710 ℃ for 1 h, and 800 ℃ for 4 h).
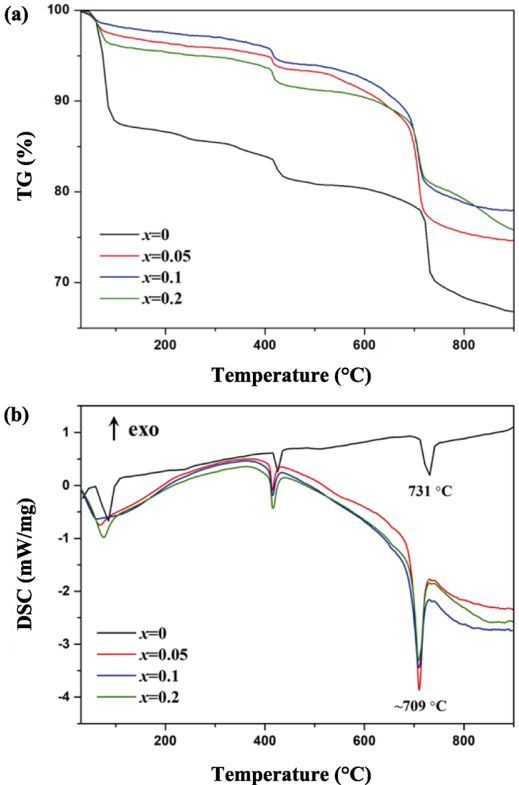
Fig. 2 TG/DSC of the precursor powders.
3. 3 Phase composition
Figure 3 shows the XRD patterns of the Li4Si1–xTixO4 (x=0, 0.05, 0.1, 0.2) samples fabricated via multistage reactive sintering. It can be seen that the undoped sample is composed of Li4SiO4, with a small amount of Li2CO3. And for the doped samples, the diffraction peaks belonging to Li4SiO4 can be obviously observed, and the weak diffraction peaks of Li2TiO3 are found. The diffraction peaks of Li4SiO4 shift to small angle with increasing Ti content, and the increase in the lattice volumes shown in Table 1 indicates the insertion of Ti4+ in the Li4SiO4 structure (the ionic radii of Si4+ and Ti4+ are 0.42 and 0.68 Å, respectively). However, the deviation of Li2TiO3 peaks does not occur, which reveals the entrance of Si into Li2TiO3 lattice is rather difficult. More secondary Li2TiO3 can be observed in x=0.2 sample. This is due to the low content of Li2CO3 in the precursor, so Li2TiO3 is retained. Previous researches show that Li2TiO3 can hardly be incorporated into the Li4SiO4 giving Li4Si1–xTixO4 solid solution [27,28]. According to the research by West et al. [29,30], Li4SiO4 and Li4TiO4 are isostructural above 700–750 ℃, the low-temperature forms of Li4SiO4 and Li4TiO4 exhibit considerable mutual solid solubility, and the limit of substituting Ti for Si is about 50%. Hence, the possible formation process of Li4Si1–xTixO4 in this work can be concluded as: Li2TiO3 reacts with Li2CO3 to form Li4TiO4 (Eq. (3)) [31], and subsequently incorporates into Li4SiO4 to generate solid solution (Eq. (4)) [32].
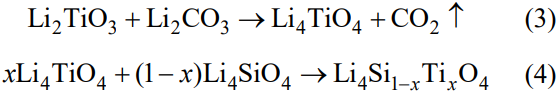
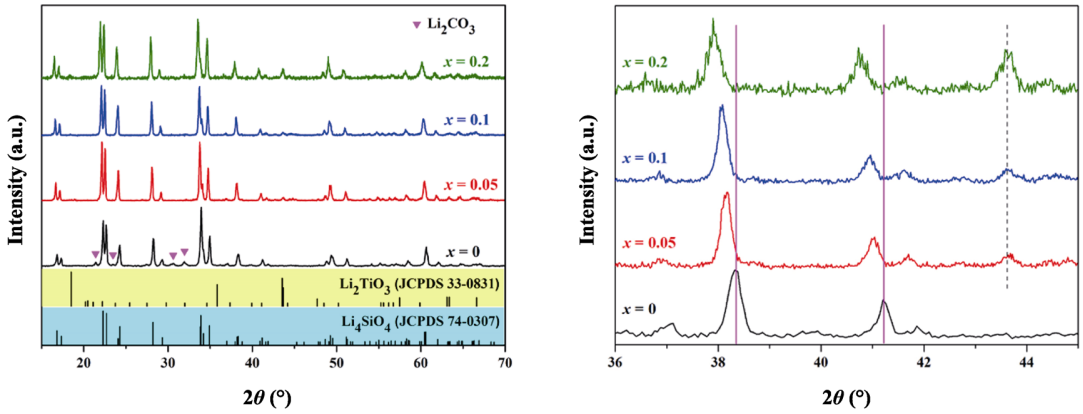
Fig. 3 XRD patterns of Li4Si1–xTixO4.
Table 1 Lattice parameters obtained using a WPF Refinement in JADE 6 Software
3. 4 Morphology of ceramic pebbles
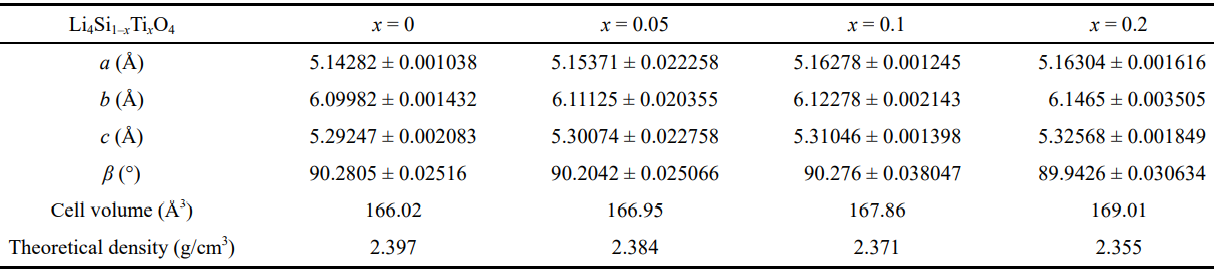
Figure 4 shows the cross-section SEM micrographs of Li4Si1–xTixO4 pebbles (x=0, 0.05, 0.1, 0.2). It can be seen that the Li4SiO4 sample is made of spherical particles and displays the fracture features of intergranular fracture, which indicates the intergranular bonding is weak. In contrast, the Li4Si1–xTixO4 samples mainly display irregular particles, and stable polygonal structure can be seen (pointed out by red arrows), which indicates that addition of Ti promotes the grain growth. The migration of interface under the interface driving force is responsible for the growth of polygonal grains. Furthermore, the grain size increases with increasing Ti content. It has been reported that the grain growth of Li4SiO4 pebbles is controlled by the lattice diffusion (volume diffusion) at about 850 ℃ [33]. As discussed above, Ti substitution increases the lattice diffusion, i.e., the activation energy for grain growth decreases and grains grow more easily for the Ti doped samples under the same sintering conditions. Additionally, the proportion of transgranular fracture rises with increasing Ti doping content (pointed out by yellow arrows), suggesting the enhancement of the grain boundary cohesion. The transgranular fracture feature predicts the enhanced mechanical strength.
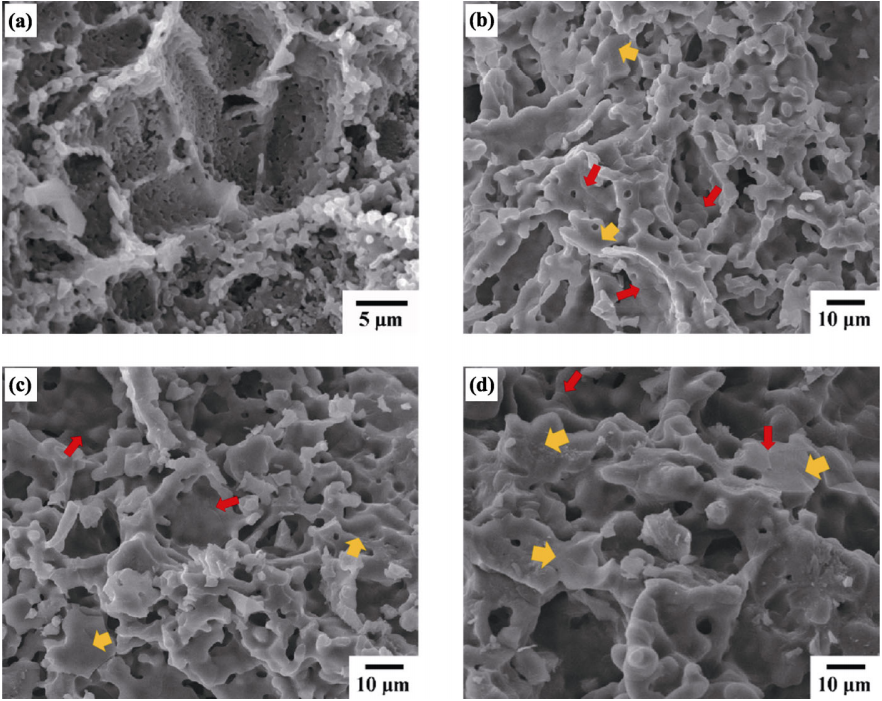
Fig. 4 Cross-section SEM micrographs of Li4Si1–xTixO4 pebbles fabricated via multistage reactive sintering: (a) x=0; (b) x=0.05; (c) x=0.1; (d) x=0.2. The enlarged micrographs are given in Figs. S1–S3 in the Electronic Supplementary Material (ESM).
EDS mapping was performed on the Li4Si0.9Ti0.1O4 sample to depict the distribution of Ti and Si elements. As shown in Fig. 5, Ti and Si elements are detected within the grains, confirming the formation of solid solution. However, the aggregation of Ti element can be found in some regions (marked with red circles). Combined with the XRD results, it can be deduced the small-sized Li2TiO3 particles exist as second phase.
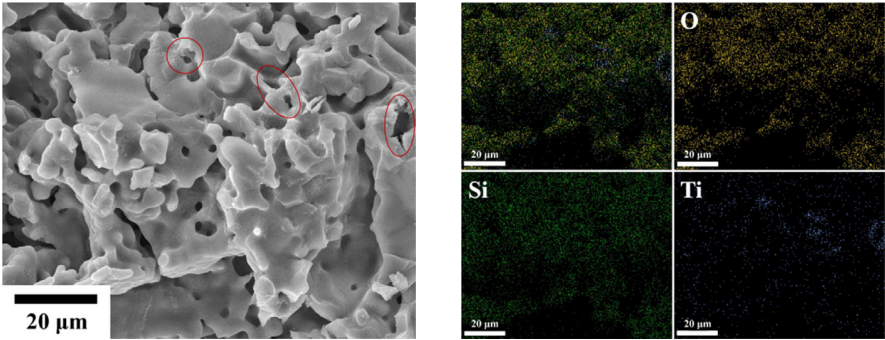
Fig. 5 SEM micrographs of Li4Si1–xTixO4 with Ti content x = 0.1 and corresponding EDS mapping of Si, Ti, and O elements.
3. 5 Crush load, density, and grain size
The ceramic pebbles need high crush load to prevent the breaking and fragmentation, which lead to plugging of purge circuits and diminished heat transfer [24]. The
dependence of crush load, density, and grain size on Ti doping amount is illustrated in Fig. 6. The density of the ceramic pebbles is 79.88%T.D. (Li4SiO4), 78.45%T.D.
(x=0.05), 79.65%T.D. (x=0.1), and 80.98%T.D. (x=0.2), respectively. The average grain size is 0.80 μm, 3.21 μm, 4.09 μm, and 5.73 μm, respectively. The crush load is increased to 27 N (x=0.05), 31 N (x=0.1), 52 N (x=0.2) compared to 11 N (Li4SiO4). It can be seen that the addition of Ti promotes the grain growth, but has relatively little effect on densification of ceramic pebbles. The ceramic densification is depended on the competition between grain boundary migration and pores removal. The results show that the effect of Ti doping on grain boundary migration prevails. And this is the reason why the density of Ti doped samples is not significantly improved compared to that of Li4SiO4
sample.
Fig. 6 Dependence of crush load, density, and grain size on Ti doping content.
Grain size and porosity are critical factors affecting the strength of ceramics, which can be expressed by the empirical equation [34]: S = kG–ae–bP, where S is the strength, G is the grain size, P is the porosity, b is a constant related to pore shape, and k, a are positive constants. The Li4Si1–xTixO4 samples have a comparable density and larger grain size, but higher crushing strength, compared to the Li4SiO4 sample. The reasons for this phenomenon are, on one side, with increasing Ti content, the lattice distortion increases and the deformation resistance of the matrix is increased, thereby resulting in a more significant solid solution strengthening effect. On the other side, second phase Li2TiO3 contained in the Li4Si1–xTixO4 samples also makes a huge contribution for the enhanced strength as the crushing strength of Li2TiO3 is better than that of Li4SiO4. It also should be noted that strength of ceramic pebbles is also affected by flaws, sphericity, and impurities.
3. 6 Thermal conductivity
Thermal conductivity k can be calculated by multiplying the bulk density ρ, the thermal diffusivity α, and the specific heat cp.

Figure 7 shows the dependence of the thermal diffusivity, specific heat, and thermal conductivity on the temperature and Ti doping amount. The relative densities of the pellets are 84.10% (x=0), 80.16% (x=0.05), 81.53% (x=0.1), 81.57% (x=0.2), respectively. It can be seen that the specific heat of the Ti doped samples is more or less the same, and is higher than that of the Li4SiO4 sample for all temperatures. The thermal diffusivity and thermal conductivity do not increase monotonically with the increase of Ti content,
and the x=0.05 sample has the best thermal conductivity and thermal diffusivity. Compared with the reported Li4+xSi1–xAlxO4 [17], the prepared Li4Si1–xTixO4 in this work exhibits lower thermal conductivity. There may be two reasons: (1) Heat conduction of Li4SiO4 can be carried out through phonons and carriers. The formation of interstitial
lithium, as the result of aluminum addition, contributes to the thermal conductivity. (2) Generally, thermal conductivity decreases with increasing porosity and is very sensitive to impurities. Hence, the presence of pores and Li2TiO3 may be detrimental to the thermal conductivity. Even so, the thermal conductivity of Li4SiO4 is obviously improved through the substitution of Ti (the best performance is not necessarily the most doped sample), it is foreseen that solid solution ceramics should be a good candidate for advanced tritium breeders.
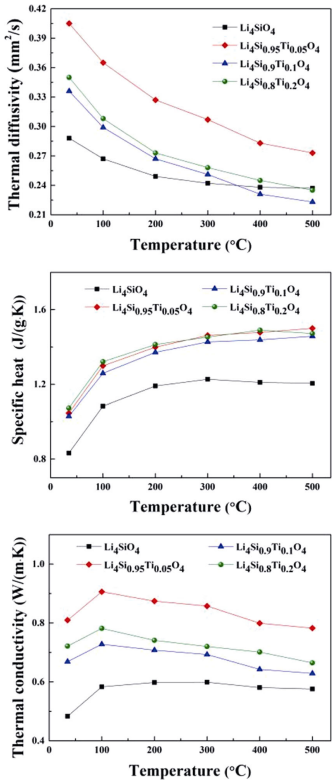
Fig. 7 Thermal diffusivity, specific heat, and thermal conductivity of Li4Si1–xTixO4 samples (x=0, 0.05, 0.1, 0.2).
3. 7 Ion conductivity
Tritium diffusion process can be envisaged as lithium-ion migration which acts as the charge carrier in the ceramics [22,35]; the tritium release behavior can be evaluated by measuring the conductivity of breeder materials. Figure 8 is the impedance spectra of the samples recorded at room temperature in the frequency range of 100–106 Hz. The equivalent circuit is composed of a resistance R1 in series with a component consisting of another resistance R2 in parallel to a constant phase element (CPE). The depressed semicircle is present in the plot, the right intercept of the semicircle with the real axis corresponds to the bulk resistance (grain interior resistance plus grain boundary resistance). The ion conductivity can be calculated in accordance with the relation:

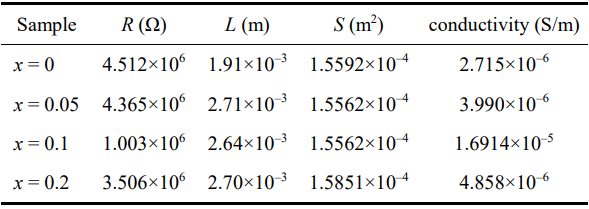
where σ is the ion conductivity, L is the sample thickness, R is the bulk resistance, and S is the area of the electrode. The calculated ion conductivity is illustrated in Table 2. It can be seen that substitution of Ti for Si causes an increase in ionic conductivity, possibly indicating the improvement in tritium release performance. The insertion of Ti4+ enlarges the lattice size of the Li4SiO4-type structure (Li4SiO4 with a monoclinic structure (space group P2/1) contains SiO4 tetrahedral elements, in which Li floats around the
silicon–oxygen tetrahedron), i.e., the size of migration channels of Li+ increases [36]. Moreover, since Ti–O bond is stronger than Si–O bond, the Li–O bond interaction in Li4SiO4 structure is decreased when the Si atom is replaced by Ti atom, and thus the ionic conductivity is improved [37]. However, the ionic conductivity decreases with increasing x from 0.1 to 0.2, and this is probably due to the increased proportion of second-phase Li2TiO3. According to the research by Tanigawa et al. [38], lithium orthosilicate has better conductivity than lithium titanate, and the electrical conductivity of Li4SiO4 is about two orders larger than that of Li2TiO3 at 702 ℃.
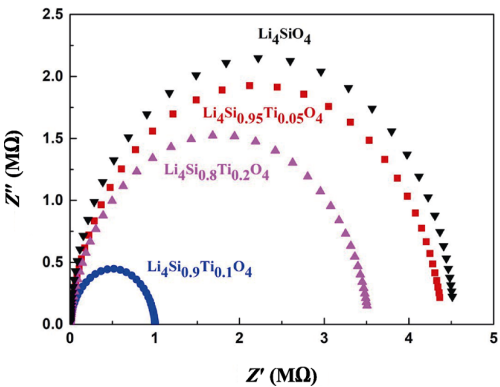
Fig. 8 AC impedance spectra for Li4Si1–xTixO4 samples
Table 2 Conductivity of Li4Si1–xTixO4 samples
3. 8 Thermal cycling
It is important to study the microstructure and crushing strength changes during the thermal cycling tests. As shown in Figs. 9 and 10, the grain size of Li4SiO4 sample increases following thermal cycles, small-sized pores merge with each other, and coarse grains with a transgranular fracture morphology can be observed. EDS analyses indicate both fine grains and coarse grains in Fig. 10(a2) are Li4SiO4 (Figs. S4 and S5 in the ESM), confirming the secondary growth of grains. The crush load increases slightly after 9 cycles (Fig. 11(a)), which is ascribed to the change of fracture mode and the decrease of pores. However, due to the presence of intracrystalline pores and cracks, the crush load decreases after 12 cycles.
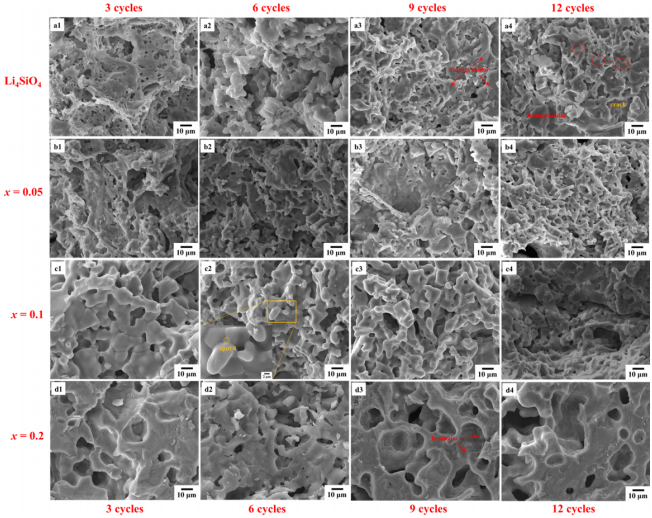
Fig. 9 Cross-section SEM micrographs of samples after thermal cycling.
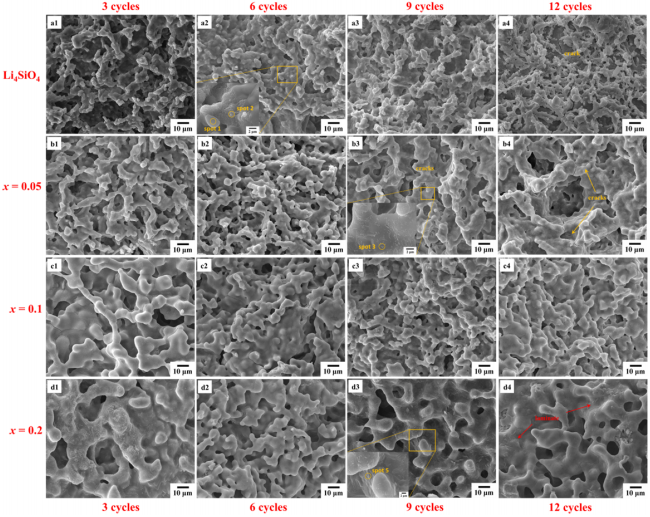
Fig. 10 Surface SEM micrographs of samples after thermal cycling.
For the Li4Si0.95Ti0.05O4 sample, no obvious changes in the microstructure and the crush load after 6 thermal cycles (Fig. 11(b)). Many tiny particles precipitate on the grain boundaries and the surface of grains (Fig. 10(b3)). EDS analysis (see Fig. S6 in the ESM) shows that Si/Ti ratio is lower than the designed value (viz. 8.3 vs. 19), suggesting the segregation of Ti toward the grain surface during long-time thermal cycling. This can lead to the slight decrease of strength after 9 cycles. In general, the crush load remains the same within the accuracy.
With prolonging the thermal cycling, the microstructure of Li4Si0.9Ti0.1O4 sample changes significantly and the strength decreases rapidly (Fig. 11(c)). EDS analysis confirms the tiny particles as titanium-rich (Fig. S7 in the ESM). The atom ratio of Si:Ti is close to 2.5:1 which is much lower than the designed value. It is reasonable to assume that the segregation becomes more serious with the increase of Ti doping content. Furthermore, a great amount of intracrystalline pores can be observed after 12 cycles. As a result, the crush load decreases to less than 20 N, revealing inferior durability of the Li4Si0.9Ti0.1O4 sample.
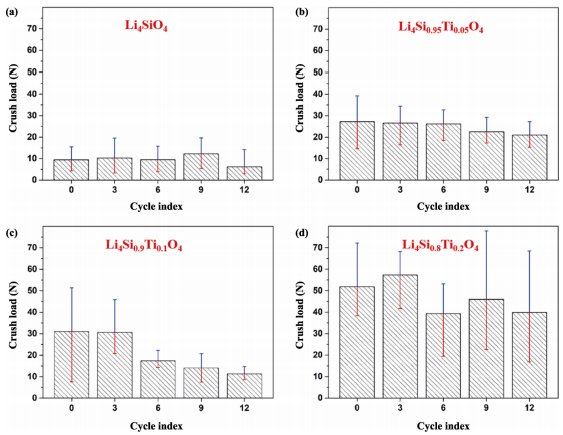
Fig. 11 Relationship between the crush load and thermal cycles: (a) Li4SiO4 pebbles, (b) Li4Si0.95Ti0.05O4 pebbles, (c) Li4Si0.9Ti0.1O4 pebbles, (d) Li4Si0.8Ti0.2O4 pebbles.
For the Li4Si0.8Ti0.2O4 sample, laminate structure mainly composed of Ti and O can be seen clearly after 9 cycles (Fig. S8 in the ESM). EDS mapping also indicates Ti with high concentration within the laminate grains (Fig. S9 in the ESM). It suggests the formation of titanate. Although the microstructure of the Li4Si0.8Ti0.2O4 sample changes slightly after the thermal cycling, it still exhibits a high crush load (Fig. 11(d)). Since many factors can affect the strength, such as grain size, porosity, pore size and distribution, sphericity, the content and size of second-phases, humidity, etc., the variation in strength with thermal cycling periods is irregular.
To sum up, the moderate amount of Ti doping can improve the mechanical and physical properties and thermal cycling stability of Li4SiO4. Compared with Al-doped Li4SiO4 [17,19,20], Ti doping plays a more significant role in enhancing crushing strength, whilst Al doping contributes more to the improvement of conductivity. Tritium release experiments conducted by Zhao et al. [18] revealed that Li4.2Si0.8Al0.2O4 had lower tritium release temperature and potentially better irradiation resistance compared to Li4SiO4. The tritium release performance and irradiation resistance of Li4Si1–xTixO4 solid solution are also worthy of expectation (Ti has lower neutron activation than Al). Since the thermal cycling stability of Li4+xSi1–xAlxO4 pebbles has not been reported, we cannot make a comparison yet. View from the current studies, solid solution materials should have unique advantages in advanced tritium breeding materials, although more deep studies are still required to further understand the influence of various doping elements.
4 Conclusions
The Li4Si1–xTixO4 ceramic pebbles were successfully prepared by proprietary hydrothermal process and multistage reactive sintering. The formation of Li4Si1–xTixO4 solid solution may be resulted from the incorporation of Li4TiO4 into Li4SiO4. Ti doping can promote the grain growth of Li4SiO4. The crush load of the Li4SiO4 pebbles is improved significantly by doping Ti (both solid solution Ti and second phase Li2TiO3 contribute to the crush load), and it increases almost four times by increasing x from 0 to 0.2. The thermal and ionic conductivity do not increase monotonically with increasing Ti content, and the best values are obtained when x=0.05 and x=0.1, respectively. Thermal cycling tests display that the Li4Si1–xTixO4 samples still have higher crush load compared with the Li4SiO4 sample, despite Ti segregation in some samples after undergoing thermal cycling. With the increase of Ti doping content, the segregation becomes more serious. In terms of thermal stability, the optimal Ti doping amount should be x=0.05. As titanium has low neutron activation behavior, Li4Si1–xTixO4 solid solution may have good application prospect in the field of solid tritium breeders.
Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 51802257), Natural Science Foundation of Shaanxi Provincial Department of Education (18JK0570), and China Postdoctoral Science Foundation (2019M663788).
Electronic Supplementary Material
Supplementary material is available in the online version of this article at https://doi.org/10.1007/s40145-020-0419-0.
References
[1] Xiang M, Wang C, Zhang Y, et al. Research progress of fabrication process for solid state tritium breeders. Adv Ceram 2016, 37: 241–252.
[2] Konishi S, Enoeda M, Nakamichi M, et al. Functional materials for breeding blankets—Status and developments. Nucl Fusion 2017, 57: 092014.
[3] Feng KM, Wang XY, Feng YJ, et al. Current progress of Chinese HCCB TBM program. Fusion Eng Des 2016, 109–111: 729–735.
[4] Johnson CE, Noda K, Roux N. Ceramic breeder materials: Status and needs. J Nucl Mater 1998, 258–263: 140–148.
[5] Ying A, Akiba M, Boccaccini LV, et al. Status and perspective of the R&D on ceramic breeder materials for testing in ITER. J Nucl Mater 2007, 367–370: 1281–1286.
[6] Li JG, Wan YX. Present state of Chinese magnetic fusion development and future plans. J Fusion Energ 2019, 38: 113–124.
[7] Hoshino T. Pebble fabrication of super advanced tritium breeders using a solid solution of Li2+xTiO3+y with Li2ZrO3. Nucl Mater Energy 2016, 9: 221–226.
[8] Hoshino T, Edao Y, Kawamura Y, et al. Pebble fabrication and tritium release properties of an advanced tritium breeder. Fusion Eng Des 2016, 109–111: 1114–1118.
[9] Hoshino T, Ochiai K, Edao Y, et al. Evaluation of tritium release properties of advanced tritium breeders. Fusion Sci Technol 2015, 67: 386–389.
[10] Wang Y, Zhou QL, Xue LH, et al. Synthesis of the biphasic mixture of Li2TiO3–Li4SiO4 and its irradiation performance. J Eur Ceram Soc 2016, 36: 4107–4113.
[11] Zhai YW, Hu J, Duan YB, et al. Characterization of tritium breeding ceramic pebbles prepared by melt spraying. J Eur Ceram Soc 2020, 40: 1602–1612.
[12] Knitter R, Kolb MHH, Kaufmann U, et al. Fabrication of modified lithium orthosilicate pebbles by addition of titania. J Nucl Mater 2013, 442: S433–S436.
[13] Kolb MHH, Knitter R, Hoshino T. Li4SiO4 based breeder ceramics with Li2TiO3, LiAlO2 and LixLayTiO3 additions, part II: Pebble properties. Fusion Eng Des 2017, 115: 6–16.
[14] Tsuchiya K, Hoshino T, Kawamura H, et al. Development of advanced tritium breeders and neutron multipliers for DEMO solid breeder blankets. Nucl Fusion 2007, 47:
1300–1306.
[15] Knitter R, Löbbecke B. Reprocessing of lithium orthosilicate breeder material by remelting. J Nucl Mater 2007, 361: 104–111.
[16] Wang M, Xiang MQ, Zhang YC. Fabrication and characterization of Li4SiO4 ceramic pebbles doped with Y2O3 and Nb2O5. Solid State Phenom 2018, 281: 28–33.
[17] Zhao LJ, Long XG, Chen XJ, et al. Design, synthesis and characterization of the advanced tritium breeder: Li4+xSi1–xAlxO4 ceramics. J Nucl Mater 2015, 467: 911–916.
[18] Zhao LJ, Long XG, Peng SM, et al. Tritium release In Li4SiO4 and Li4.2Si0.8Al0.2O4 ceramics. J Nucl Mater 2016, 482: 42–46.
[19] Xiang MQ, Zhang YC, Zhang Y, et al. Preparation, performances and reaction mechanism of the Li4+xAlxSi1–xO4 pebbles for advanced tritium breeders. Fusion Eng Des
2017, 116: 17–23.
[20] Gong YC, Li JJ, Yang SH, et al. Improvement of crushing strength and thermal conductivity by introduction of heteroelement Al into Li4SiO4. Ceram Int 2019, 45: 24564–24569.
[21] Saito Y, Asai T, Ado K, et al. Ionic conductivity of Li+ ion conductors, Li4.2MxSi1–xO4 (M: B3+, Al3+, Ga3+, Cr3+, Fe3+, Co2+, Ni2+). Solid State Ionics 1990, 40–41: 34–37.
[22] Dash U, Sahoo S, Parashar SKS, et al. Effect of Li+ ion mobility on the grain boundary conductivity of Li2TiO3 nanoceramics. J Adv Ceram 2014, 3: 98–108.
[23] Roux N, Hollenberg G, Johnson C, et al. Summary of experimental results for ceramic breeder materials. Fusion Eng Des 1995, 27: 154–166.
[24] Roux N, Johnson C, Noda K. Properties and performance of tritium breeding ceramics. J Nucl Mater 1992, 191–194: 15–22.
[25] Wu XW, Wen ZY, Xu XG, et al. Synthesis and characterization of Li4SiO4 nano-powders by a water-based sol–gel process. J Nucl Mater 2009, 392: 471–475.
[26] Johnson CE, Kondo T, Roux N, et al. Fabrication, properties, and tritium recovery from solid breeder materials. Fusion Eng Des 1991, 16: 127–139.
[27] Dang C, Yang M, Gong YC, et al. A promising tritium breeding material: Nanostructured 2Li2TiO3–Li4SiO4 biphasic ceramic pebbles. J Nucl Mater 2018, 500: 265–269.
[28] Leys O, Kolb MHH, Pucci A, et al. Study of lithium germanate additions to advanced ceramic breeder pebbles. J Nucl Mater 2019, 518: 234–240.
[29] Dubey BL, West AR. Crystal chemistry of Li4XO4 phases: X = Si, Ge, Ti. J Inorg Nucl Chem 1973, 35: 3713–3717.
[30] West AR. Ionic conductivity of oxides based on Li4SiO4. J Appl Electrochem 1973, 3: 327–335.
[31] Chen RC, Shi QW, Yang M, et al. Microstructure and phase evolution of Li4TiO4 ceramics pebbles prepared from a nanostructured precursor powder synthesized by hydrothermal method. J Nucl Mater 2018, 508: 434–439.
[32] West AR. Ionic conductivity of oxides based on Li4SiO4. J Appl Electrochem 1973, 3: 327–335.
[33] Xiang MQ, Zhang YC, Zhang Y, et al. Grain growth behavior of Li4SiO4 pebbles fabricated by agar method for tritium breeder. Fusion Eng Des 2016, 112: 513–519.
[34] Knudsen FP. Dependence of mechanical strength of brittle polycrystalline specimens on porosity and grain size. J Am Ceram Soc 1959, 42: 376–387.
[35] Zhou QL, Xue LH, Wang Y, et al. Preparation of Li2TiO3 ceramic with nano-sized pores by ultrasonic-assisted solution combustion. J Eur Ceram Soc 2017, 37: 3595–3602.
[36] Adnan SBRS, Mohamed NS. Effects of Sn substitution on the properties of Li4SiO4 ceramic electrolyte. Solid State Ionics 2014, 262: 559–562.
[37] Ortiz-Landeros J, Gómez-Yáñez C, Palacios-Romero LM, et al. Structural and thermochemical chemisorption of CO2 on Li4+x(Si1–xAlx)O4 and Li4–x(Si1–xVx)O4 solid solutions. J Phys Chem A 2012, 116: 3163–3171.
[38] Tanigawa H, Tanaka Y, Enoeda M, et al. Thermal conductivity measurement with silica-coated hot wire for Li4SiO4 pebble bed. J Nucl Sci Technol 2009, 46: 553–556.
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.