Non-oxide ceramic materials generally refer to metal compounds and non-metal compounds mainly composed of B, C, N, etc. Due to their high melting point, high strength, excellent corrosion resistance, and thermal shock resistance, they have broad application prospects in industries such as metallurgy, chemical industry, machinery, aerospace, military, and energy.
Fine-grained, high-purity, and highly active powder is the basis for preparing high-performance non-oxide ceramics. The synthesis methods of non-oxide ceramic powders mainly include metal element reaction method, carbothermal reduction method, sol-gel method, chemical vapor reaction method, and solvent thermal synthesis method. The advantages and disadvantages of using these methods to synthesize non-oxide ceramic powders are as follows: The main advantages of molten salt method include low synthesis temperature, short reaction time, small particle size, high purity, controllable structure, simple reaction process, easy control, no special equipment, low cost, and easy industrial production.
In recent years, research on the preparation of non-oxide ceramic powders using the molten salt method has gradually become a hot topic. This article briefly reviews the research progress of the molten salt method in the synthesis of non-oxide ceramic powders in recent years.
1 Introduction to the Molten Salt Method
1.1 Principles of the Molten Salt Method
The molten salt method refers to adding salts with relatively low melting points that do not react with the reactants into the reaction system. When the reaction temperature exceeds the melting point of these salts, the molten salt provides a liquid phase environment for the synthesis of the target product. After the reaction is completed and the temperature drops to room temperature, the molten salt is removed by washing and filtration with a suitable solvent to obtain the target product powder.
The main functions of molten salt in the reaction process are twofold: as a solvent for the reactants and as a reaction medium. According to the different solubility and diffusion of the reactants in the liquid salt, the reaction mechanism of the molten salt method can be summarized into two types:
(1) Template synthesis mechanism. As shown in Figure 1(a), the solubility of component B in the molten salt is greater than that of component A. After B is dissolved, it diffuses to the surface of A and reacts to produce product C.
(2) Dissolution-precipitation mechanism. As shown in Figure 1(b), components A and B have a certain solubility in the molten salt, and they react to produce product D in a molecular or atomic mixed state.
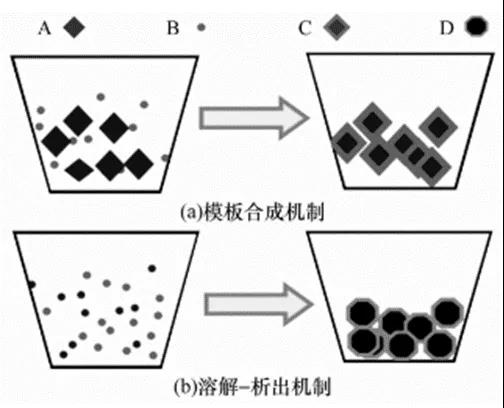
Figure 1 Mechanism diagram of the molten salt method
1.2 Principles for Selecting Molten Salts
The type of salt has a significant impact on the morphology and properties of the powder prepared by the molten salt method. When selecting the type of salt, the following principles are usually followed:
(1) The salt should have as low a melting point and as high a boiling point as possible.
(2) The salt should have as low a viscosity as possible to facilitate the transport of solutes and energy.
(3) The reactants should have a sufficiently high solubility in the molten salt.
(4) The salt should be easily soluble in a certain liquid solvent, such as water, acid or alkaline solution, which has no corrosive effect on the product.
(5) The solid solubility in the product should be as small as possible to avoid the molten salt from entering the product as an impurity.
(6) The salt should not form stable impurity phases over as wide a temperature range as possible.
(7) The salt should have low volatility, no corrosiveness and toxicity.
2 Research on the Synthesis of Non-Oxide Ceramic Powders by the Molten Salt Method
In recent years, research on the synthesis of non-oxide ceramic powders such as carbides, nitrides, and borides using the molten salt method has gradually become a hot topic in this field. Based on the experimental results of some researchers, the following summary can be obtained:
Translation:
2.1.1 One-Dimensional Carbide-Based Carbide Powders
Due to the insolubility of carbon materials in molten salt media, one-dimensional carbide powders prepared by molten salt synthesis mechanism have the advantages of the same morphology as carbon template materials, low synthesis temperature, and short reaction time.
2.1.2 Carbide Coatings
Carbon materials commonly suffer from poor oxidation resistance and hydrophobicity, which affect their applications in refractory materials and high-temperature ceramics. Coating the surface of carbon materials with a carbide that has good hydrophilicity and excellent oxidation resistance is an effective approach.
Molten salt method has unparalleled advantages in the preparation of carbide coatings. The liquid-phase environment provided by molten salt can evenly distribute elements such as Si, Ti, and Ta on the surface of carbon materials, and then produce a smooth and complete carbide coating through surface in-situ reaction. However, when the coating is too thick, there will be gaps between the coating and the substrate, and the bonding strength between them will decrease, leading to cracking and peeling of the coating. On the other hand, when the coating is too thin, it cannot effectively protect the carbon substrate. Therefore, the key to using molten salt method to coat the surface of carbon materials is to choose the right molten salt and control the process parameters to prepare a carbide coating with appropriate thickness.
2.3 Nitrides
Although some researchers have successfully attempted to prepare nitride ceramic powders using molten salt method, there are still few reports on this approach, and the biggest challenge is the lack of suitable nitrogen sources. Because conventional nitrogen-containing substances are prone to decompose and lose nitrogen gas at high temperatures, N2 is generally used as the nitrogen source for preparing nitrides by molten salt method. However, N2 is difficult to fully contact with the reactants in the molten salt medium, which cannot fully exploit the advantages of the molten salt method. Therefore, the key to using molten salt method to prepare nitrides is to find low-cost, highly reactive nitrogen-containing substances that will not decompose at high temperatures, or how to prevent nitrogen-containing substances in the molten salt medium from being lost at high temperatures.
2.3 Borides
Although there have been some studies on the synthesis of borides (MB2) by molten salt method, they are still limited. The methods for synthesizing boride powders can be summarized into three categories:
1) Direct chemical reaction between metal and boron;
2) Direct reaction between metal chlorides and boron hydrides;
3) Co-reaction of metal oxides, boron-containing compounds, and reducing agents.
All three processes are solid-state reactions and suffer from the problem of uneven mixing of raw materials. The liquid-phase environment provided by molten salt method can achieve full mixing of raw materials at the molecular or atomic level, greatly reducing the energy required for the reaction from a kinetic perspective. Therefore, the molten salt method has great potential in the preparation of boride powders.
3 Conclusion
The liquid-phase reaction environment provided by molten salt method effectively solves the problem of long diffusion paths in solid-state reactions, so it can effectively reduce the reaction temperature and shorten the reaction time. The particle size of non-oxide ceramic powders prepared by this method can be reduced to sub-micron or even nano-scale, with uniform distribution, high purity, and low agglomeration. Currently, there are many studies on the preparation of carbide ceramic powders by molten salt method, but research on nitride and boride powders is still limited.
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.