Abstract: The non-stoichiometric Li3Mg2Sb1-xO6 (0.05 ≤ x ≤ 0.125) compounds have been prepared via the mixed oxide method. The influences of Sb nonstoichiometry on the sintering behavior, microstructure, phase composition along with microwave dielectric performances for Li3Mg2Sb1–xO6 ceramics were studied. Combined with X-ray diffraction (XRD) and Raman spectra, it was confirmed that phase composition could not be affected by the Sb nonstoichiometry and almost pure phase Li3Mg2SbO6 was formed in all compositions. Appropriate Sb-deficiency in Li3Mg2SbO6 not only lowered its sintering temperature but also remarkably improved its Q×f value. In particular,
non-stoichiometric Li3Mg2Sb0.9O6 ceramics sintered at 1250 ℃/5 h owned seldom low dielectric constant εr = 10.8, near-zero resonant frequency temperature coefficient τf = -8.0 ppm/℃, and high quality factor Q×f = 86,300 GHz (at 10.4 GHz). This study provides an alternative approach to ameliorate its dielectric performances for Li3Mg2SbO6-based compounds through defect-engineering.
Keywords: microwave dielectric properties; ceramics; sintering; antimony compounds
1 Introduction
Nowadays, the high-speed advancement in the 5th-generation communication industry has prompted a massive demand for dielectric materials owning outstanding dielectric properties in the high-frequency region [1]. To satisfy the particular criteria, the microwave dielectric material must own following essential parameters: low dielectric constant (εr) to shorten signal transition time, small resonant frequency temperature coefficient (τf) to enhance thermal stabilization, and high quality factor (Q×f ) or low dielectric loss to enhance frequency selectivity [2]. It is still a challenge for one material owing εr, τf, and Q×f simultaneously since the majority of microwave dielectric ceramics commonly own low εr but large negative τf values [3]. To obtain near-zero τf, one effective approach is to prepare a solid solution or composite ceramics [4]. However, this method could usually deteriorate the host ceramics’ microwave dielectric properties to some extent owing to the unexpected secondary phase or ion diffusion [5,6]. Therefore, new material systems owing above parameters simultaneously need to be explored and investigated [7].
In 1982, Castellanos et al. [8] reported an orthorhombic Li3Mg2SbO6 (LMS) compound. Recently, the LMS compound has received much attention due to its potential application in fields such as luminescence and microwave dielectric materials [9–13]. Zhang et al. [13] reported the microwave dielectric properties of Li3Mg2(Nb1–xSbx)O6 (0.02 ≤ x ≤ 0.08) ceramics. We firstly reported the microwave dielectric performances (Q×f = 49,000 GHz, τf = -18.0 ppm/℃) of nominal composition LMS ceramics prepared with a
mixed oxide method [9]. Yet its poor sinterability (dehiscence) and low Q×f inhibit its practical application, which is connected with the secondary phase SbOx [9]. Lately, we doped modified solid-state reaction method, which not only effectively suppressed the secondary phase SbOx but also improved the sinterability and dielectric characterizations of LMS ceramics [10]. The above-modified process not only prolongs the preparation period, but also enhances the cost. Recently, many researchers have demonstrated that the sintering behavior and dielectric performances can be ameliorated through introducing non-stoichiometric composition in some material systems [14-17]. However, the effects of non-stoichiometry on Li3Mg2SbO6 ceramics have not yet been reported. In the current paper, the influences of Sb-site nonstoichiometry on
the sintering behavior, phase constitution combined with microwave dielectric characterizations of Li3Mg2Sb1–xO6 compounds have been studied.
2 Experimental
The non-stoichiometric Li3Mg2Sb1–xO6 (0.05 ≤ x ≤0.125) were synthesized via solid-state ceramics processing. The reagents of Sb2O3 (99.0%, Guo-Yao Co., Ltd., Shanghai, China), MgO (99.99%, Mountain Development Center, Beijing, China), and Li2CO3 (98.0%, Guo-Yao Co., Ltd., Shanghai, China) were used as staring materials. According to non-stoichiometric Li3Mg2Sb1–xO6, the corresponding raw materials were weighed and ground for 8 h using ZrO2 balls as well as absolute ethanol as media. The resultant milled
powders were roasted under 80 ℃/5 h, and calcined at 900 ℃/4 h. After second ball-milled for 8 h, the ball-milled powders were mixed with 6% polyvinyl alcohol solution as the binder, and then granulated manually. The granules were pressed into cylindrical pucks (Ф10 mm × 6 mm) under 200 MPa. After debinding at 500 ℃ for 2 h, the pucks were fired under 1200–1275 ℃ dwelling for 5 h.
The phase constitutions of samples have been characterized using X-ray diffraction (XRD, RigakuD/ MAX2550, Tokyo, Japan) with Cu Kα radiation and Raman spectra (Jobin Yvon, Longjumeau, France) equipped with He–Ne laser (633 nm) and an output of 30 mW. X-ray photoelectron spectroscopy (XPS) measurement was carried out with a spectrometer (Axis Ultra, UK) using Al Ka (1486.6 eV) radiation. The surface morphology of sintered bodies was analyzed by adopting scanning electron microscope (SEM, Hitachi, Tokyo, Japan). The bulk densities of sintered bodies were evaluated based on Archimedes' principle. The εr and Q×f values of samples were tested through vector network analyzer (N5230A, Agilent, America) under about 10–12 GHz. The τf value for samples was calculated by Eq. (1):

where f1 and f2 denote the measured frequencies under 25 and 85 ℃, respectively.
3 Results and discussion
The refined XRD patterns and refinement parameters for Li3Mg2Sb1–xO6 (0.05 ≤ x ≤ 0.125) specimens heated at 1250 ℃ are displayed in Fig. 1 and Table 1, respectively. As shown in Fig. 1, the calculated XRD profiles based on Li3Co2TaO6 (ICSD #81043) structural model matched well with those of experimental ones. It indicated that for all compositional specimens, almost pure phase Li3Mg2SbO6 was formed along with trace amount of unknown phase (marked as * in Fig. 1), although the maximum 12.5 mol% Sb-site defects were introduced. Similar phenomena were also reported in other materials [18]. In addition, as seen in Table 1, the cell volume was enlarged when x increased from 0.05 to 0.125, which is due to the increased concentration of Vo caused by Sb5+ ion defects [19,20]. Figure 2 gives the refined XRD plots for Li3Mg2Sb0.9O6 specimens
heated at 1200–1275 ℃. No obvious phase constitution change or peak position shift was observed in Fig. 2, which indicated that there was no obvious change of unit cell volume. Therefore, based on the definition of packing fraction [21], there should be no change of packing fraction regardless of sintering temperature for a given composition. Compared to Ref. [9], the secondary phase in Li3Mg2SbO6-based ceramics could be effectively inhibited by introduced partial Sb-site deficiency within the crystals.

Fig. 1 Refined XRD patterns of Li3Mg2Sb1–xO6 ceramics sintered at 1250 ℃.

Fig. 2 Refined XRD patterns of Li3Mg2Sb0.9O6 ceramics under different sintering temperatures.
Table 1 Refinement parameters and reliability factors of Li3Mg2Sb1–xO6 ceramics sintered at 1250 ℃ for 5 h

The typical Raman spectra of Li3Mg2Sb1–xO6 samples under different sintering temperatures are depicted in Fig. 3. Only three Raman peaks (475, 551, and 655 cm–1) are present in all samples, which are similar to the characteristic Raman spectra of Li3Mg2SbO6 [10]. The Raman peak at 655 cm–1 can attribute to the asymmetric stretching vibration of Sb–O–Sb bond in SbO6 octahedral. The Raman peaks at 551 and 475 cm–1 are associated with the Li/Mg–O bonds vibration. In addition, there was no significant change of Raman shifts and Raman peak intensity under different sintering temperatures, which were associated with inherent microwave dielectric properties [22].Furthermore, the Raman band of SbO6 octahedron (655 cm–1) slightly shifted to lower frequency with increasing x content as seen the inset in Fig. 3. According to Refs. [15,23], the Raman shift of SbO6 octahedron depended mainly on the Sb–O bond: υ = 21349exp(–1.9176dSb-O), where υ and dSb-O represent Raman shift and Sb–O bond length, respectively. This indicated that the Raman shift was inversely proportional to the unit cell volume. Therefore, the Raman analysis confirmed that the unit cell volume of Li3Mg2Sb1–xO6 ceramics expand with increasing x content, which was well correlated with the XRD results.

Fig. 3 Characteristic Raman spectra of Li3Mg2Sb1–xO6 ceramics fired under various temperatures: (a) Li3Mg2Sb0.95O6, 1250 ℃, (b) Li3Mg2Sb0.9O6, 1200 ℃, (c)Li3Mg2Sb0.9O6, 1225 ℃, (d) Li3Mg2Sb0.9O6, 1250 ℃, (e) Li3Mg2Sb0.9O6, 1275 ℃, and (f) Li3Mg2Sb0.875O6, 1250 ℃.
Figure 4 exhibits the SEM photographs of polished and thermally etched surfaces for Li3Mg2Sb1–xO6 samples heated under different temperatures. As seen from Figs. 4(a) and 4(e), a relatively porous structure was observed for Li3Mg2Sb0.9O6 fired at 1200 ℃ and for Li3Mg2Sb0.95O6 fired at 1250 ℃, respectively. As seen from Figs. 4(a)–4(c), the amount of pores are alleviated, and mean grain size is promoted with the increment temperature. The 1250 ℃-sintered specimen (Fig. 4(c)) exhibited a dense microstructure, implying a higher dielectric performance [24]. However, for Li3Mg2Sb0.9O6 fired at 1275 ℃ and Li3Mg2Sb0.875O6 sintered at 1250 ℃, anomalous grain growth along with
pores appeared due to over-sintering, which would deteriorate the performances of samples [25]. Table 2 lists the concentrations of compositional elements, which were
conducted on grains A–C marked in Fig. 4(c) by the EDS analysis. The EDS analysis revealed that the constitution of large grains (marked A and B) is Li3Mg2SbO6, whereas the smaller and brighter grains are enriched in Mg (marked C). This is consistent with XRD results.

Fig. 4 Typical SEM photographs of polished and thermally etched surfaces for Li3Mg2Sb1–xO6 ceramics under different firing temperatures: (a) Li3Mg2Sb0.9O6, 1200 ℃, (b) Li3Mg2Sb0.9O6, 1225 ℃, (c) Li3Mg2Sb0.9O6, 1250 ℃, (d) Li3Mg2Sb0.9O6, 1275 ℃, (e) Li3Mg2Sb0.95O6, 1250 ℃, and (f) Li3Mg2Sb0.875O6, 1250 ℃.
Table 2 Energy disperse spectroscopy (EDS) data of the grains A–C marked in Fig. 4(c)

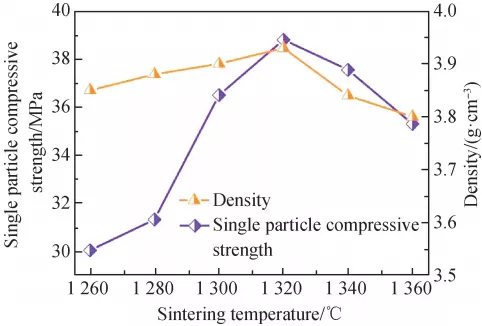
Figure 5 shows the bulk density of Li3Mg2Sb1–xO6 ceramics after heat treatment at 1200–1275 ℃. For Li3Mg2Sb0.95O6 sample, the bulk density gradually increased with increasing temperature from 1200 to 1275 ℃, indicating it has high densification sintering temperature. However, for Li3Mg2Sb0.9O6 sample, the bulk density increased gradually to the maximum at 1250 ℃ and subsequently descended. The enhancement in bulk density of Li3Mg2Sb0.9O6 sample could attribute to the reduction in porosity and grain boundaries, whereas its decrement could attribute to the anomalous grain growth, as displayed in Fig. 4. In addition, compared with Li3Mg2SbO6 ceramics [10], the low sintering temperature of Li3Mg2Sb0.9O6 ceramics could attribute to the Sb deficiency and oxygen vacancies within the crystals [26].

Fig. 5 Bulk density of Li3Mg2Sb1–xO6 ceramics following sintered at 1200–1275 ℃.
Figure 6 displays the variations in εr, τf, and Q×f of Li3Mg2Sb1–xO6 compounds with firing temperature. In general, εr is dramatically dependent on density, ionic polarizability, secondary phase, etc. [27].The relationship between εr and sintering temperature or composition of Li3Mg2Sb1–xO6 ceramics revealed a similar tendency as between density and sintering temperature or composition, as shown in Fig. 6(a). Thus, the εr value is mainly influenced by densification rather than ionic polarizability and secondary phase. In addition, the τf values of Li3Mg2Sb0.9O6 ceramics remained stable (about –8.0 ppm/℃) regardless of sintering temperatures as seen in Fig. 6(b). Both intrinsic parameters (vibration modes and packing fraction) and extrinsic parameters (density, mean grain size, phase composition, etc.) can influence the Q×f value of ceramics [28,29]. In the present ceramics, the intrinsic factors should be ignored because of no significant change of vibration mode (Fig. 3) and packing fraction as mentioned before. As illustrated in Fig. 6(c), when firing temperature varied from 1200 to 1250 ℃, the Q×f value of specimens gradually enhanced and obtained a maximum value of ~62,800 GHz for Li3Mg2Sb0.95O6 at 1250 ℃, and ~86,300 GHz for Li3Mg2Sb0.9O6 at 1250 ℃, respectively. The increased densification and average grain size could be responsible for the improvement of Q×f value [30]. Beyond this temperature, their Q×f values decreased slightly, which may be connected with the anomalous grain growth [25]. Interestingly, the
Li3Mg2Sb0.875O6 ceramics sintered below 1250 ℃ showed poor resonance, which was due to the effect of the yellow core caused by the valence change of antimony ions (Fig. 7) [31]. The resemble phenomena have also been observed in Ti-containing oxides ceramics [32,33]. However, the Li3Mg2Sb0.875O6 sample sintered at 1250 ℃ exhibited Q×f ≈ 37,400 GHz due to the absence of yellow cores. A more detailed explanation about this phenomenon is expected in further research. At a given sintering temperature of 1250 ℃, the Li3Mg2Sb0.9O6 sample exhibited superior Q×f value than the others, indicating moderate Sb-deficiency is benefited to improve the Q×f value of Li3Mg2SbO6-based ceramics. The true reason is still unknown and further research is in progress. Compared to our previous study [10], the non-stoichiometric Li3Mg2Sb0.9O6 ceramics fabricated by classical solid-state method owned comparable dielectric performances with Li3Mg2SbO6 ceramics prepared via modified two-stage process, but relatively lower sintering temperature and simple synthesis process. The comparable or even slightly enhanced Q×f value would be related to the distortion of crystal lattice caused by an appropriate Sb-site nonstoichiometry, a similar phenomenon was also reported in other ceramic systems [34–36]. Moreover, the Q×f value (86,300 GHz, at 10.4 GHz) of Li3Mg2Sb0.9O6 ceramics is 1.7 times larger than that of nominal composition Li3Mg2SbO6 ceramics (Q×f = 49,000 GHz at 11.0 GHz), which is ascribed to the absence of secondary SbOx [9].

Fig. 6 Variations in microwave dielectric properties of Li3Mg2Sb1–xO6 ceramics under different firing temperatures.

Fig. 7 Sb 3d region of XPS spectra of 1225 ℃-sintered Li3Mg2Sb0.875O6. The inset illustrates the evidence of coring in Li3Mg2Sb0.875O6 samples fired at different conditions: (a) 1225 and (b) 1250 ℃.
4 Conclusions
The non-stoichiometric Li3Mg2Sb1–xO6 (0.05 ≤ x ≤0.125) ceramics were fabricated, and their phase composition, sintering character, and dielectric properties were characterized. XRD and Raman spectrum results confirmed that Li3Mg2SbO6 without obvious secondary phase can be maintained within the compositional range of 0.05 ≤ x ≤ 0.125. The sinterability and Q×f values were tremendously improved by introducing appropriate Sb-deficiency in Li3Mg2SbO6. Especially, the nonstoichiometric Li3Mg2Sb0.9O6 ceramics sintered at 1250 ℃ simultaneously exhibited small τf of –8.0 ppm/℃ and εr of 10.8, a high Q×f of 86,300 GHz (at 10.4 GHz). The favorable combined microwave dielectric performances make it an alternative material for millimeter-wave devices.
References
[1] Zhang X, Tang B, Fang ZX, et al. Structural evolution and microwave dielectric properties of a novel Li3Mg2–x/3Nb1–2x/3TixO6 system with a rock salt structure. Inorg Chem Front 2018, 5: 3113–3125.
[2] Zhang YH, Sun JJ, Dai N, et al. Crystal structure, infrared spectra and microwave dielectric properties of novel extra low-temperature fired Eu2Zr3(MoO4)9 ceramics. J Eur Ceram Soc 2019, 39: 1127–1131.
[3] Reaney IM, Iddles D. Microwave dielectric ceramics for resonators and filters in mobile phone networks. J Am Ceram Soc 2006, 89: 2063–2072.
[4] Gu FF, Chen GH, Li XQ, et al. Structural and microwave dielectric properties of the (1−x)Li3NbO4−xCa0.8Sr0.2TiO3 thermally stable ceramics. Mater Chem Phys 2015, 167: 354–359.
[5] Zou ZY, Chen ZH, Lan XK, et al. Weak ferroelectricity and low-permittivity microwave dielectric properties of Ba2Zn(1+x)Si2O(7+x) ceramics. J Eur Ceram Soc 2017, 37: 3065–3071.
[6] Dong MZ, Yue ZX, Zhuang H, et al. Microstructure and microwave dielectric properties of TiO2-doped Zn2SiO4 ceramics synthesized through the sol-gel process. J Am Ceram Soc 2008, 91: 3981–3985.
[7] Huang CL, Tseng YW. Structure, dielectric properties, and applications of CaTiO3-modified Ca4MgNb2TiO12 ceramics at microwave frequency. J Am Ceram Soc 2011, 94: 1824– 1828.
[8] Castellanos M, Gard JA, West AR. Crystal data for a new family of phases, Li3Mg2XO6: X = Nb, Ta, Sb. J Appl Cryst 1982, 15: 116–119.
[9] Yao GG, Pei CJ, Gong Y, et al. Microwave dielectric properties of temperature stable (1 – x)Li3Mg2SbO6–xBa3(VO4)2 composite ceramics. J Mater Sci: Mater Electron 2018, 29: 9979–9983.
[10] Pei CJ, Hou CD, Li Y, et al. A low εr and temperature-stable Li3Mg2SbO6 microwave dielectric ceramics. J Alloys Compd 2019, 792: 46–49.
[11] Wang SY, Sun Q, Devakumar B, et al. Mn4+-activated Li3Mg2SbO6 as an ultrabright fluoride-free red-emitting phosphor for warm white light-emitting diodes. RSC Adv 2019, 9: 3429–3435.
[12] Zhong JS, Chen X, Chen DQ, et al. A novel rare-earth free red-emitting Li3Mg2SbO6: Mn4+ phosphor-in-glass for warm w-LEDs: Synthesis, structure, and luminescence properties. J Alloys Compd 2019, 773: 413–422.
[13] Zhang P, Wu SX, Xiao M. Effect of Sb5+ ion substitution for Nb5+ on crystal structure and microwave dielectric properties for Li3Mg2NbO6 ceramics. J Alloys Compd 2018, 766: 498–505.
[14] Guo WJ, Zhang J, Luo Y, et al. Microwave dielectric properties and thermally stimulated depolarization of Al-doped Ba4(Sm,Nd)9.33Ti18O54 ceramics. J Am Ceram Soc 2019, 102: 5494–5502.
[15] Li B, Zheng JG, Li W. Enhanced effect of vanadium ions non-stoichiometry on microwave dielectric properties of Ca5Co4V6+xO24 ceramics. Mater Chem Phys 2018, 207: 282–288.
[16] Belous A, Ovchar O, Jancar B, et al. The effect of nonstoichiometry on the microstructure and microwave dielectric properties of the columbites A2+Nb2O6. J Eur Ceram Soc 2007, 27: 2933–2936.
[17] Li JM, Fan CG, Cheng ZX, et al. Influence of Zn nonstoichiometry on the phase structure, microstructure and microwave dielectric properties of Nd(Zn0.5Ti0.5)O3 ceramics. J Alloys Compd 2019, 793: 385–392.
[18] Pang LX, Zhou D, Yue ZX. Temperature independent low firing [Ca0.25(Nd1–xBix)0.5]MoO4 (0.2 ≤ x ≤ 0.8) microwave dielectric ceramics. J Alloys Compd 2019, 781: 385–388.
[19] Pan WG, Cao MH, Qi JL, et al. Defect structure and dielectric behavior in SrTi1–x(Zn1/3Nb2/3)xO3 ceramics. J Alloys Compd 2019, 784: 1303–1310.
[20] Muhammad R, Khesro A. Influence of A-site nonstopichiometry on the electrical properties of BT-BMT. J Am Ceram Soc 2017, 100: 1091–1097.
[21] Kim ES, Chun BS, Freer R, et al. Effects of packing fraction and bond valence on microwave dielectric properties of A2+B6+O4 (A2+:Ca,Pb,Ba; B6+:Mo,W) ceramics. J Eur Ceram Soc 2010, 30: 1731–1736.
[22] Wang Y, Tang TL, Zhang JT, et al. Preparation and microwave dielectric properties of new low-loss NiZrTa2O8 ceramics. J Alloys Compd 2019, 778: 576–578.
[23] Hardcastle FD, Wachs IE. Determination of molybdenumoxygen bond distances and bond orders by Raman spectroscopy. J Raman Spectrosc 1990, 21: 683–691.
[24] Wu SP, Chen DF, Jiang C, et al. Synthesis of monoclinic CaSnSiO5 ceramics and their microwave dielectric properties. Mater Lett 2013, 91: 239–241.
[25] Song JB, Song KX, Wei JS, et al. Microstructure characteristics and microwave dielectric properties of calcium apatite ceramics as microwave substrates. J Alloys Compd 2018, 731: 264–270.
[26] Bian JJ, Song GX, Yan K. Structure and microwave dielectric properties of Ba1+x[(Co0.7Zn0.3)1/3Nb2/3]O3(–0.015 ≤ x ≤ 0.015). J Eur Ceram Soc 2007, 27: 2817– 2821.
[27] George S, Sebastian MT. Synthesis and microwave dielectric properties of novel temperature stable high Q, Li2ATi3O8 (A = Mg, Zn) ceramics. J Am Ceram Soc 2010, 93: 2164–2166.
[28] Gurevich VL, Tagantsev AK. Intrinsic dielectric loss in crystals. Adv Phys 1991, 40: 719–767.
[29] Wang KG, Zhou HF, Liu XB, et al. A lithium aluminium borate composite microwave dielectric ceramic with low permittivity, near-zero shrinkage, and low sintering temperature. J Eur Ceram Soc 2019, 39: 1122–1126.
[30] Kai C, Li CC, Xiang HC, et al. Phase formation and microwave dielectric properties of BiMVO5 (M = Ca, Mg) ceramics potential for low temperature co-fired ceramics application. J Am Ceram Soc 2019, 102: 362–371.
[31] Kim SS, Na HG, Kwon YJ, et al. Synthesis and room-temperature NO2 sensing properties of Sb2O5 nanowires. Met Mater Int 2015, 21: 415–421.
[32] Freer R, Azough F. Microstructural engineering of microwave dielectric ceramics. J Eur Ceram Soc 2008, 28: 1433–1441.
[33] Pullar RC, Penn SJ, Wang XR, et al. Dielectric loss caused by oxygen vacancies in titania ceramics. J Eur Ceram Soc 2009, 29: 419–424.
[34] Surendran KP, Sebastian MT, Mohanan P, et al. Effect of nonstoichiometry on the structure and microwave dielectric properties of Ba(Mg0.33Ta0.67)O3. Chem Mater 2005, 17: 142–151.
[35] Zhang TW, Zuo RZ. Effect of Li2O–V2O5 addition on the sintering behavior and microwave dielectric properties of Li3(Mg1–xZnx)2NbO6 ceramics. Ceram Int 2014, 40: 15677–15684.
[36] Desu SB, O'Bryan HM. Microwave loss quality of BaZn13Ta2/3O3 ceramics. J Am Ceram Soc 1985, 68: 546–551.
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.