Abstract: Duplex α/β-SiAlON ceramic cutting inserts (30α:70β) were synthesized by microwave sintering. The effects of solid solution parameters (m, n, z), synthesis temperature, and amount of excess Y2O3 synthesis additive on phase assemblage, microstructure, mechanical properties, and cutting performance were systematically investigated. It was found that increasing m value could improve the formation of α phase while high z value over 1.0 resulted in the dissolution of α phase into β phase and intergranular phase. Increasing the amount of excess Y2O3 could promote densification and elongated β grain growth; however, the excess Y2O3 amount above 4 wt% resulted in substantial crystallization of M'SS phase, thus declining the mechanical properties and wear resistance. The microwave-synthesized α/β-SiAlON cutting insert with modified parameters (m = 1.7, n = 1.0, z = 0.7, and 3 wt% excess Y2O3) was obtained with optimal comprehensive properties, whose tool life was found to increase by approximately 75% in high-speed milling of Inconel 718 superalloy compared to the commercial α/β-SiAlON cutting insert.
Keywords: α/β-SiAlON; microwave; ceramic cutting insert; solid solution parameter; Y2O3 synthesis additive
1 Introduction
SiAlON ceramics are substitutional solid solutions based on silicon nitride (Si3N4). Basically, there are two main crystal structures of SiAlON ceramics for engineering applications, namely α-SiAlON and β-SiAlON. The general formulas of α- and β-SiAlON are MxSi12−(m+n)Al(m+n)OnN16−n (x = m/v, v indicates the valence of the modifying cation M (Y3+, Ca2+, etc.)) and Si6−zAlzOzN8−z (0 < z ≤ 4.2), respectively, where m and n values represent the Al–N and Al–O substitutions in α-Si3N4 crystal lattice, respectively, and z value represents the Al–O substitution in β-Si3N4 crystal lattice. In general, α-SiAlON formed as equiaxed grains exhibits higher hardness than β-SiAlON; in contrast, β-SiAlON formed as elongated hexagonal grains possesses enhanced fracture toughness compared to α-SiAlON [1]. Therefore, by changing the overall composition of raw powder and varying α/(α+β) ratios, duplex α/β-SiAlON ceramic materials with comprehensive properties can be successfully prepared [2–4].
Duplex α/β-SiAlON ceramics have attracted significant research interest as cutting tool materials in machining of difficult-to-cut materials such as nickel-based superalloys because of their good wear resistance and outstanding chemical stability at elevated temperatures [5]. According to Refs. [6,7], the cutting speeds of α/β-SiAlON ceramic cutting tools can be up to 300–500 m·min−1 in turning nickel-based superalloys and 800–1000 m·min−1 when milling, which are 10 times those of cemented carbide tools. Nevertheless, rapid wear still occurs for α/β-SiAlON ceramic cutting tools during the high-speed machining nickel-based superalloys [8]. Moreover, the inefficiency and high cost of SiAlON ceramic cutting tools synthesized by the commonly used conventional sintering processes such as pressureless sintering, hot-pressed sintering, and hot isostatic pressing sintering limit the development and wide application prospects of SiAlON ceramic cutting tools.
Microwave synthesis of SiAlON ceramic tools is considered to be an effective approach to solve the above-mentioned problems. During microwave sintering process, samples can be directly heated through the interaction with electromagnetic wave such that the heating rate reaches 30–100 ℃·min–1 and the holding time gets reduced to 0–10 min, which are more efficient parameters than those employed in conventional sintering (5–10 ℃·min–1, 60–240 min) [9,10]. Owing to the volumetric fast heating, microwave-prepared ceramics can acquire better densification and mechanical properties [11,12]. Furthermore, as a type of pressureless sintering, microwave sintering offers great potential for mass production of complex-shaped cutting inserts; however, pressure sintering methods cannot provide this benefit. This makes microwave synthesis more attractive for low-cost industrial production of ceramic cutting inserts.
In addition to the synthesis technology, modification of the overall compositions and synthesis conditions could be an effective way to enhance the cutting performance [12–15]. Noteworthy, for α/β-SiAlON ceramics, a deep correlation was observed among the solid solution parameters, the synthesis additives amount, the densification behavior, and the microstructural evolution [16–20]. According to Refs. [21–25], the m and n values have great impact on the stability of α phase; the z value is an effective parameter that significantly influences the wear resistance, crystallization of intergranular phases, and microstructural characteristics; and the synthesis additive amount affects the α-SiAlON phase stability and intergranular phase. Thus, it can be speculated that changing the overall compositions of solid solution parameters (m, n, z) and synthesis additives leads to the variation in the liquid level and solid solution reaction during synthesis process, thus influencing the phase assemblage, microstructure, oxidation resistance, and mechanical and machining properties of α/β-SiAlON ceramic cutting inserts [26–28]. Among reported studies on SiAlON, the microwave synthesis of α/β-SiAlON ceramics (especially cutting tool application) has rarely been carried out [29]. Undeniably, systematically exploration of overall compositions is indispensable for obtaining high-quality duplex α/β-SiAlON ceramic cutting inserts by synthesized microwave sintering.
In order to prolong the service life, the optimization of mechanical properties is significantly important because the higher hardness can improve the resistance to penetration of abrasive particle and thereby reduce the cutting depth, and at the same time, the higher fracture toughness can reduce the ratio of microcutting to microploughing [30–33]. Importantly, the nature and crystallization of secondary phase(s) and microstructure (grain size, porosity, etc.) also play a significant role in cutting tool application of SiAlON ceramics. Aluminum-containing melilite phase (M'SS phase, Y2Si3−xAlxO3+xN4−x) has been considered as a better phase for SiAlON ceramics as it displays excellent high temperature stability due to its very high nitrogen content [34]. Acikbas et al. [35] reported that α/β-SiAlON cutting tools with crystalline grain boundary (M'SS phase) and coarsened grain size exhibited better cutting performance than that with amorphous phase because the increased crystallinity and coalescence of intergranular phase reduced the tendency to undergo the chemical reaction. On the other hand, Aucote and Foster [36] tested the cutting performance of SiAlON cutting inserts in machining nickel-based superalloy Incoloy 901, and the wear resistance was found to increase with tool material grain size at high speeds.
In the present study, small batch microwave synthesis of duplex α/β-SiAlON ceramic cutting inserts was realized. The effects of solid solution parameters (m, n, z), synthesis temperature, and amount of excess Y2O3 synthesis additive were systematically investigated. Finally, after modifying these parameters, the wear resistance of microwave-sintered α/β-SiAlON ceramic cutting inserts, compared with that of the commercial α/β-SiAlON ceramic cutting insert in dry milling of nickel-based superalloy Inconel 718, was studied.
2 Experimental
2. 1 Green body preparation
The composition of the duplex α/β-SiAlON with different m, n, z values was designed by Eqs. (1) and (2):
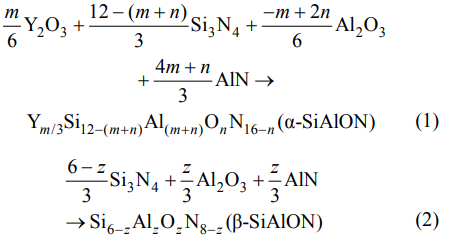
The mass ratio of α/β was kept constant at 30/70. In addition to the Y2O3 dissolved in the α phase, excess Y2O3 with amount of 1–5 wt% was added as synthesis additive.
Figure 1(a) displays the preparation process of the green body. The raw powders were composed of α-Si3N4 (99.99%, 500 nm; Shanghai Chaowei Nano Technology Co., Ltd., China), α-Al2O3 (99.99%, 200 nm; Shanghai Chaowei Nano Technology Co., Ltd., China), AlN (99.99%, 1 μm; Shanghai Chaowei Nano Technology Co., Ltd., China), and Y2O3 (99.99%, 50 nm; Shanghai Chaowei Nano Technology Co., Ltd., China). The mixed powders were poured into an Al2O3 jar with isopropanol alcohol and Si3N4 balls, and then the ball milled in a planetary ball mill (Model QM-3SP2, Nanjing, China) for 2 h at a speed of 250 r·min−1. The ball-to-powder mass ratio was 8:1. The 2 wt% PVA was added in the slurries as binder. After ball milling, the slurries were dried in a vacuum drying oven, ground by agate grinding bowl, and sieved through a 100-mesh. Finally, the green bodies were compacted into a cylinder with the diameter of 16 mm under a uniaxial pressure of 150 MPa.
2. 2 Microwave synthesis
Figure 1(b) displays the diagrammatic sketch of the microwave insulation box. BN crucible was placed in the center of the mullite insulation box, covered with mullite insulation cotton. Three green bodies were placed plat in the center of BN crucible, covered with SiC susceptor to help with the synthesis. Figure 1(c) displays the diagrammatic sketch of the microwave synthesis system. The heat insulation box was put on the base of a microwave atmosphere furnace (XO-5kW, ATPIO, China) with a rotation speed of 5 r·min−1. Through the thermometer pore of the microwave insulation box, the real time temperature of the samples can be measured by the infrared thermometer. Before synthesis, air in the furnace chamber was pumped out and replaced by N2. In the initial stage of synthesis process, the microwave atmosphere furnace was operated at minimum power of 900 W during which the heating rate was about 50–100 ℃·min−1. Then, the heating rate was kept constant at 30 ℃·min−1 between 470 and 1400 ℃ and 15 ℃·min−1 after 1400 ℃ until reaching the desired temperatures (1690–1750 ℃). The holding time was 10 min. After that, the samples were naturally cooled to room temperature in the furnace. The microwave-synthesized α/β-SiAlON cutting inserts are shown in Fig. 1(d).
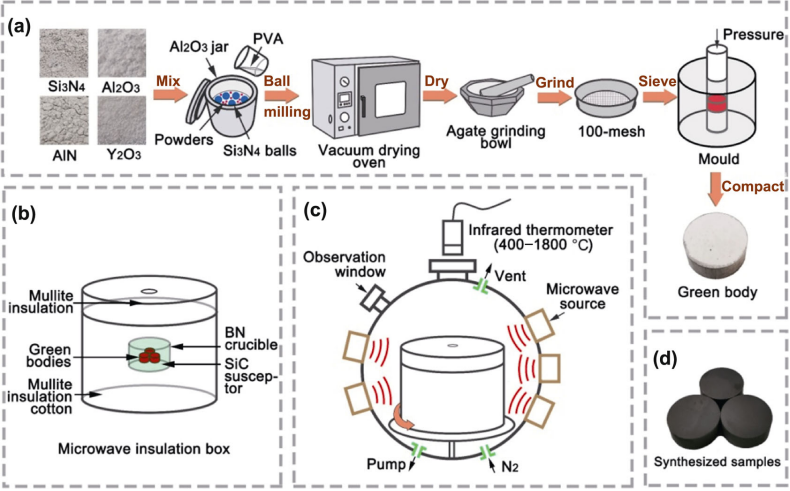
Fig. 1 (a) Preparation process of green body, (b) diagrammatic sketch of the microwave insulation box, (c) diagrammatic sketch of the microwave synthesis system, and (d) microwave-synthesized α/β-SiAlON cutting inserts.
2. 3 Characterization
The bulk density of the synthesized samples was calculated by the Archimedes' method. The Vickers hardness was measured through indentation method under a load of 98 N and holding time of 15 s. Fracture toughness measurement was based upon the Vickers indentation method. The equation of the fracture toughness of the samples is as follows [37]:
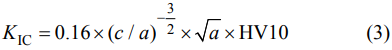
where KIC is the fracture toughness (MPa·m1/2), a and c are half the length of the indentation diagonal (mm) and the crack (mm), respectively (c/a > 2.5), and HV10 is the Vickers hardness (GPa). The microstructure and energy dispersive X-ray (EDX) analysis of the samples were detected by the scanning electron microscope (SEM, Hitachi S-4800, Japan) and the transmission electron microscope (TEM, JEOL JEM-F200, Japan).
The X-ray diffraction analyses (XRD, Bruker-AXS D8 Advance, USA) were conducted to detect the α/(α+β) ratio and the types of other phases. The used PDF cards of α-SiAlON, β-SiAlON, and α-Si3N4 were #42-0251, #48-1615, and #41-0360, respectively. Besides, the labels of M'ss phase (aluminum-containing melilite phase, Y2Si3−xAlxO3+xN4−x) and 21R phase (AlN polytypoid, SiAl6O2N6) were referenced to Refs. [38,39]. The α/(α+β) ratio was calculated from the XRD patterns by Eq. (4):
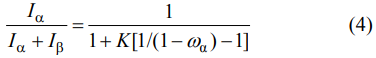
where ωα is the mass ratio of α/(α+β), Iα is the intensity of the stronger peak of α(102) and α(210), Iβ is the intensity of the stronger peak of β(101) and β(210), and K is the combined proportionality constant calculated by the equation: K = Kα/Kβ, where Kα is 0.518 for α(102) reflection and 0.544 for α(210) reflection, and Kβ is 0.518 for β(101) reflection and 0.544 for β(210) reflection [40].
2. 4 Machining test
Figure 2 shows the schematic diagram of the machining test. The wear resistance of microwave-synthesized duplex α/β-SiAlON ceramic milling inserts and commercial duplex α/β-SiAlON ceramic milling insert (Kennametal KYS30, RNGN120400EGN) was evaluated by rough milling Inconel 718 superalloy (speed νc = 800 m·min−1, depth ap = 1.5 mm, and feed rate ƒz = 0.12 mm·z−1). The diameter and thickness of the round insert are 12.70 and 4.76 mm, respectively, which was clamped on one tooth of Kennametal KCRA63Z06S22RN12 indexable face mill. The tool parameters of the face mill are shown in Fig. 2(b). The flank wear was measured by a digital microscope (ISM-PM200S, Insize, China) during testing in intervals of 1 cut (cutting length: 150 mm, width: 33 mm). The allowable maximum flank wear of insert (VB) was 1.5 mm in this test, and the insert was identified as invalid when VB exceeded this value.
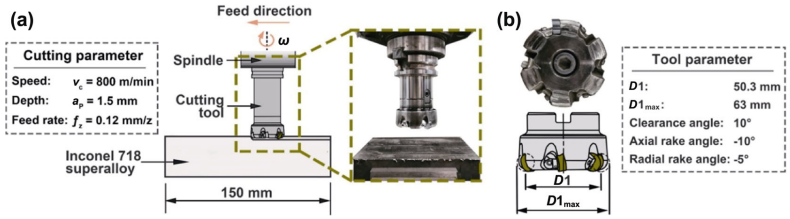
Fig. 2 (a) Schematic diagram of the machining test and (b) tool parameters. The effective diameter (D1) is 50.3 mm. The maximum diameter (D1max) is 63 mm. The clearance angle, axial rake angle, and radial rake angle are 10°, −10°, and −5°, respectively.
3 Results and discussion
3. 1 Orthogonal experiment (m, n, z values and synthesis temperature)
Orthogonal experiment of four factors at three levels was carried out first for selecting the general scope of appropriate m, n, z values and the synthesis temperature. The detailed experimental design and results are presented in Table 1, and the corresponding XRD patterns are shown in Fig. 3. The m value of α-SiAlON was set at 1.0 (1), 1.35 (2), and 1.7 (3), and its n value was set at 1.0 (1), 1.35 (2), and 1.7 (3). The z value of β-SiAlON was set at 0.4 (1), 0.7 (2), and 1.0 (3), and the synthesis temperature was set at 1690 ℃ (1), 1720 ℃ (2), and 1750 ℃ (3). Excess Y2O3 (2 wt%) was added as synthesis additive. The main objective of the orthogonal experiment was to obtain α/β-SiAlON ceramics with designed composition (α/(α+β) ratio = 30%) without residual raw powder phase, and the second objective was to screen the influencing parameters for obtaining well optimized mechanical properties (hardness and fracture toughness).
Table 1 illustrates that the detected α/(α+β) ratio was calculated between 2% and 29% (< 30%), in which some α phase was converted into β phase. Moreover, residual α-Si3N4 phase was detected in samples S1, S5, S6, and S9, among which the intensity for S1 was relatively strong (Fig. 3). This indicated that the solid solution reaction was not complete, which could have resulted from the low synthesis temperature (1690−1720 ℃) or low liquid content of these components [21]. For the samples synthesized at 1750 ℃ (S3, S4, and S8), α-Si3N4 was dissolved, and higher bulk density over 3.22 g·cm−3 was obtained. For the secondary phases, M'SS phase was detected in all samples, while 21R phase was detected in samples S3, S5, and S7 with high z value of 1.0.
Table 1 Orthogonal design and corresponding bulk density, phase assemblage, and mechanical properties of the α/β-SiAlON ceramicsa
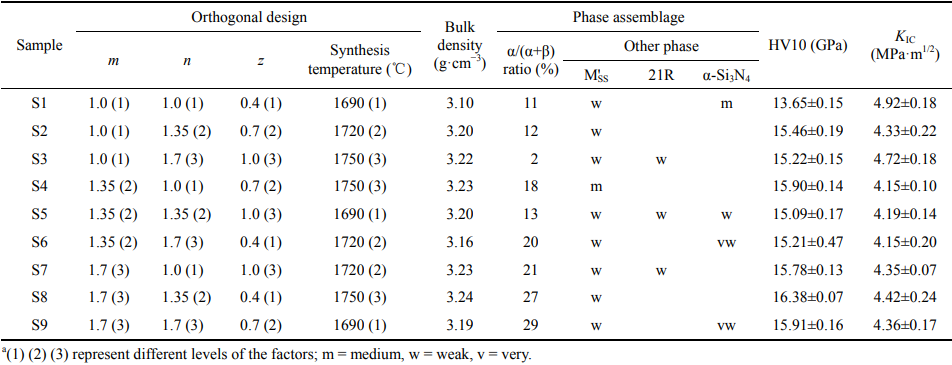
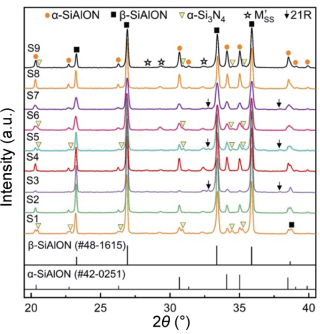
Fig. 3 XRD patterns of the orthogonal designed α/β-SiAlON ceramics (M'SS: aluminum-containing mellite phase, Y2Si3−xAlxO3+xN4−x; 21R: AlN polytypoid, SiAl6O2N6).
Table 2 lists the range analysis results of α/(α+β) ratio, hardness, and fracture toughness of the orthogonal experiment. Considering α/(α+β) ratio as the index, the R value (R indicates the range value of each factor) of m value was much greater than those of other factors. With the increase in the m value from 1.0 to 1.7, the average result of α/(α+β) ratio was evidently promoted from 8.3% to 25.7%, which was closer to the setting value of 30%. This phenomenon could be attributed to the increasing amount of Y2O3 in the raw powder composition (x = m/3, Eq. (1)). With higher amount of Y2O3, more rare-earth (RE) cations could be incorporated into the α-SiAlON structure, thus improving the formation and stability of α phase [24]. On the other hand, the variations of other parameters with the average result of α/(α+β) ratio were not obvious. Notably, when the z value was increased to 1.0, a decline in α/(α+β) ratio was observed from 19.7% to 12.0%. This might be attributed to the excessive oxygen content induced by the high z value, which damaged the stability of α-SiAlON phase [41]. For obtaining SiAlON ceramics with high α/(α+β) ratio, low z value of 0.4–0.7 was appropriate.
Table 2 Range analysis results of hardness, fracture toughness, and α/(α+β) ratio of the orthogonal experimentb
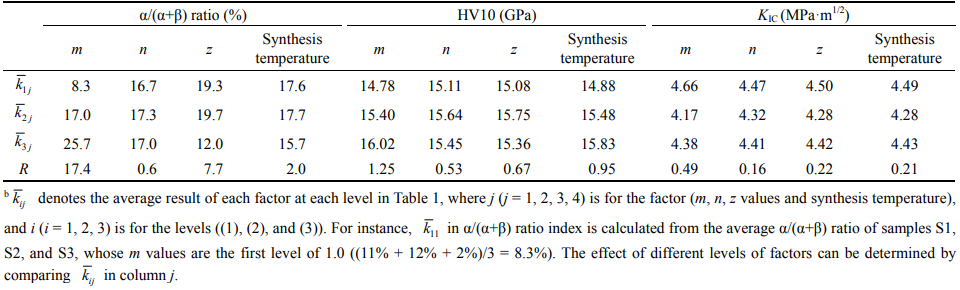
Considering hardness as the representative index, the R values of m, n, z values, and synthesis temperature were 1.25, 0.53, 0.67, and 0.95 GPa, respectively. It indicates that m value and synthesis temperature played more important roles, and the average result of hardness was found to increase with these two factors. The amelioration of hardness induced by m value could be attributed to increasing α/(α+β) ratio. However, for synthesis temperature, the improvement could be due to the reduced porosity. Table 1 presents that the average density of sample increases with the synthesis temperature. With the increase in average density, the porosity decreases; as a result, ceramic materials acquire higher hardness [42]. Considering fracture toughness as the index, it was observed that the R value of m value was greater than those of other factors, but it was still small (0.49 MPa·m1/2) and the variations were irregular for all factors. This indicated that these factors did not exhibit significant impact on the fracture toughness within the setting range. According to the results, high m value of 1.7 was required for improving the formation of α phase and synthesis temperature of 1750 ℃ was necessary for enhancing the densification and mechanical properties. The impacts of n and z values are not evident, and the further systematic exploration is needed.
3. 2 Effects of z value on phase assemblage, microstructure, and mechanical properties of α/β-SiAlON ceramics
In α/β-SiAlON ceramics, n and z values represent the Al–O substitution in Si–N of α- and β-SiAlON, respectively. Noteworthy, when α/(α+β) ratio is 30%, the effect of increasing n value by 0.7 in the composition of raw powder is consistent with the effect of increasing z value by 0.14 (e.g., the raw powder composition of Y–α/β-SiAlON with n value of 1.7 and z value of 0.4 is equal to that with n value of 1.0 and z value of 0.54 for the same m value). Consequently, it can be inferred that the impact of n and z values on α/β-SiAlON ceramics is similar in a certain range. Notably, β ratio was higher in this research; therefore, the effects of z value were studied, and the z value was set at 0.4, 0.54, 0.7, 0.84, 1.0, and 1.14. The m value was kept at 1.7, the n value was kept constant at 1.0, and the excess Y2O3 amount was 2 wt%. The synthesis temperature was set at 1750 ℃.
Figure 4(a) shows the XRD patterns of the α/β-SiAlON ceramics with different z values. Weak α-Si3N4 peaks were detected in the sample with z value of 0.4 since the oxygen content was low, and these peaks disappeared with the increase in the z value. For the secondary phases, M'SS phases were detected in all samples and 21R phases were detected in the samples with z value exceeding 1.0 (the same as the orthogonal experiment results shown in Fig. 3). Moreover, substantial crystallization was observed as z value increased to 1.14, which could be attributed to the high oxygen content. Figure 4(b) shows the variation in α/(α+β) ratio with z value. Under z value of 0.4–0.84, the α/(α+β) ratio varied between a narrow range of 27%–36% (close to setting value of 30%). However, when z value was increased to 1.0 and 1.14, the α/(α+β) ratio evidently decreased to 15% and 14%, respectively. Correspondingly, the XRD patterns shown in Fig. 4(a) indicate that the intensities of α-SiAlON phase (the peaks at 2θ of 34.0° and 35.0°) were also obviously reduced.
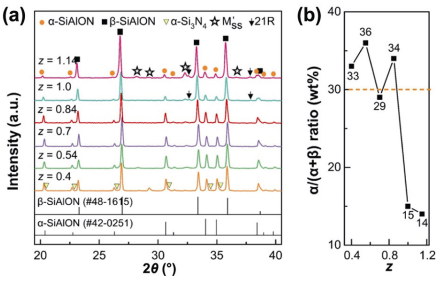
Fig. 4 (a) XRD patterns (M'SS: aluminum-containing melilite phase, Y2Si3-xAlxO3+xN4-x; 21R: AlN polytypoid, SiAl6O2N6), and (b) α/(α+β) ratio of the α/β-SiAlON ceramics with different z values (m = 1.7, n = 1.0, synthesized at 1750 ℃)
Carman et al. [38] reported the presence of a reversible thermodynamic transformation relationship between α- and β-SiAlON phases: α + liquid ↔ β + M'SS + 21R. They reported that the long-time heat-treatment at 1450 ℃ for 72 h led to α → β transformation and the reverse β α transformation was observed under subsequent heat treatment at 1800 ℃ for 4 h, which was accompanied with the change of M'SS phase and 21R phase. The observation in this research when z value exceeded 1.0 was similar to the α → β transformation reaction, that is, the α/(α+β) ratio was reduced and the crystallization of M'SS and 21R phases was enhanced. In α/β-SiAlON ceramics, the stability of the α phase is related to the liquid phase and temperature, and will dissolute while the conditions appear to permit significant diffusion [39]. When z value was 1.0, the liquid content exceeded critical level, and this resulted in the dissolution of α phase. Acikbas and Kara [27] also studied the effect of z value (0.3–1.1) on the phase evolution of α/β-SiAlON prepared at 1840–1890 ℃; however, the α/(α+β) ratio barely changed and no 21R phase was detected. This observation was different, but it is still in agreement with our explanation that the α-SiAlON phase is more stable at high temperatures according to the β → α transformation.
Figure 5 shows the microstructures of the α/β-SiAlON ceramics with different z values obtained under SEM-BSE (backscattered electron, BSE) mode. The microstructure presented three different contrasting phases, namely, α-SiAlON (light gray), β-SiAlON (dark gray), and intergranular phase (white area). The α-SiAlON phase exhibited the existence of grains with low aspect ratio, while the β-SiAlON phase mainly consisted of grains with high aspect ratio. With increasing z value, the grain size of β-SiAlON grains became relative coarse (Figs. 5(e) and 5(f)); however, the changes in α-SiAlON grains were not evident. Under high z value of 1.0 and 1.14, the area of β-SiAlON was obviously facilitated, which was consistent with the variation of α/(α+β) ratio shown in Fig. 4. Moreover, the intergranular phase was generally distributed around the SiAlON grains. In the sample with higher z value, there should be more aluminum in the intergranular phase such that the Si–N bonds in M'SS phase (Y2Si3−xAlxO3+xN4−x) can be progressively replaced with Al–O bonds. This can lead to the increase of oxygen content, which in turn can improve the oxidation resistance of the material [2,43].
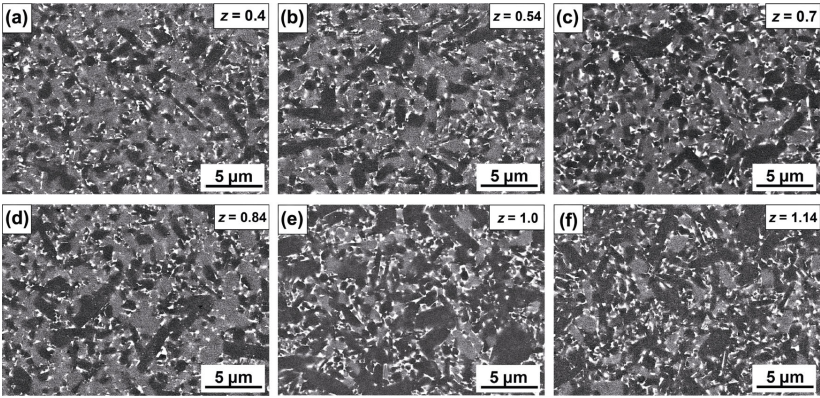
Fig. 5 Microstructure of α/β-SiAlON ceramics with different z values (m = 1.7, n = 1.0, synthesized at 1750 ℃) obtained under SEM–BSE mode: (a) z = 0.4, (b) z = 0.54, (c) z = 0.7, (d) z = 0.84, (e) z =1.0, and (f) z = 1.14.
Figure 6 shows the bulk density, hardness, and fracture toughness variations with z value. The bulk density measured in the range of 3.23–3.24 g·cm−3 was close to the theoretical density based on Refs. [26,44]. The hardness and fracture toughness of the α/β-SiAlON ceramics exhibited an overall declining trend with the increase of z value; however, the change was not significant. With the increase in the z value from 0.4 to 0.84, the mechanical properties of samples narrowly varied in a small range (hardness: 16.35–16.76 GPa, fracture toughness: 4.28–4.84 MPa·m1/2) because of their similar α/(α+β) ratio (29%–36%). When z value exceeded 1.0, the hardness was declined to 16.02±0.12 GPa due to the dissolution of α phase (Fig. 4(b)). Moreover, with the increase in the z value to 1.14, both hardness and fracture toughness declined to 15.46± 0.07 GPa and 3.87±0.07 MPa·m1/2, respectively. This could be attributed to the substantial crystallization of the intergranular phases (Fig. 4) as their mechanical properties were relatively poor (hardness: 12–15 GPa, fracture toughness: 3–5 MPa·m1/2 [45]). Although the α/β-SiAlON ceramics with low z value possessed better mechanical properties, it was reported that SiAlON ceramics with low z value (e.g., 0.2–0.3) exhibited a short tool life in machining of superalloy due to chemical wear [46]. Besides, α/β-SiAlON ceramics with low z value were also difficult to synthesize because of the detection of residual α-Si3N4 (Fig. 4(a)). Thus, it is speculated that the z value of 0.54–0.84 should be appropriate for microwave synthesis of α/β-SiAlON ceramics.
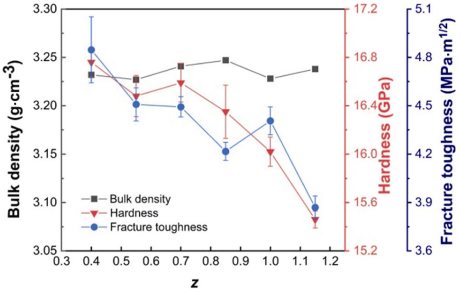
Fig. 6 Bulk density, hardness, and fracture toughness variations with z value.
3. 3 Effects of the amount of excess Y2O3 synthesis additive on phase assemblage, microstructure, mechanical properties, and cutting performance of α/β-SiAlON ceramic milling inserts
Furthermore, the effect of the amount of excess Y2O3 synthesis additive was investigated. Based on the above-mentioned results, the m, n, z values were set at 1.7, 1.0, and 0.7, respectively, and the synthesis temperature was set at 1750 ℃. The α/β-SiAlON ceramics containing the excessive amount of Y2O3 of 1–5 wt% were fabricated, and denoted as Y1–Y5, respectively.
Figure 7 shows the XRD patterns of the samples Y1–Y5 and α/(α+β) ratio varied with the excessive amount of Y2O3. Analysis of the XRD patterns indicates that M'SS phase was detected as the only intergranular phase in all the materials. The intensity of M'SS phase (peak at 2θ of 32.3°, colored area shown in Fig. 7(a)) was relatively weak in samples Y1, Y2, and Y3, and significantly enhanced when the excessive amount of Y2O3 exceeded 4 wt%. On the other hand, the α/(α+β) ratio decreased to 20% when the amount of Y2O3 reached 4 wt%, whereas the α/(α+β) ratios of the other samples were found to be close to the setting value of 30% (±4%). In high-temperature synthesis of α/β-SiAlON ceramics, two positions are suggested for Y2O3: incorporation into α-SiAlON structure or in intergranular phase. As discussed in Section 3.1, increasing amount of Y2O3 induced by increasing m value remarkably promoted the α/(α+β) ratio to the setting value (Table 2), whereas the crystallization level of intergranular phase was low (Table 1). It could be inferred that Y2O3 preferentially tended to get incorporated into α-SiAlON, and the samples Y1–Y3 might have higher x value than the designed value since their α/(α+β) ratios reached 30% after modifying the m value to 1.7. For sample Y4, the Y2O3 amount reached a critical level and substantial crystallization of M'SS phase was observed (Fig. 7(a)). After the devitrification of M'SS phase, the available RE cations in the liquid were consumed; therefore, the α/β-SiAlON shifted to β-SiAlON. With the increase in added amount of Y2O3 to 5 wt%, the α/(α+β) ratio increased again to the setting value.
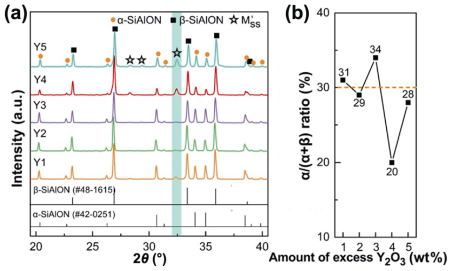
Fig. 7 (a) XRD patterns (M'SS: aluminum-containing melilite phase, Y2Si3–xAlxO3+xN4–x), and (b) α/(α+β) ratio of the α/β-SiAlON ceramics with different amounts of excess Y2O3 (m = 1.7, n = 1.0, z = 0.7, synthesized at 1750 ℃).
Figure 8 shows the microstructures of α/β-SiAlON ceramics with different amounts of excess Y2O3 synthesis additive. The α-SiAlON phase exhibited the existence of grains with low aspect ratio (light gray). The β-SiAlON grains (dark gray) were relatively fine in sample Y1 and many β-SiAlON grains appeared to be equiaxed with low aspect ratio (Fig. 8(a)). Comparative analysis indicates that more elongated β-SiAlON grains were observed in samples Y2–Y5 and the β-SiAlON grains were relatively coarsened with increasing amount of excess Y2O3. This was in agreement with Herrmann et al. [22] that the RE-containing liquid phase showed a decisive role for elongated grain growth at high synthesis temperature. Moreover, the intergranular phase (white area) was distributed around SiAlON grains. In accordance with the increase of excess Y2O3, more white area of the RE-rich melilite phase was observed (Figs. 8(d) and 8(e)), illustrating the facilitation of intergranular phase.
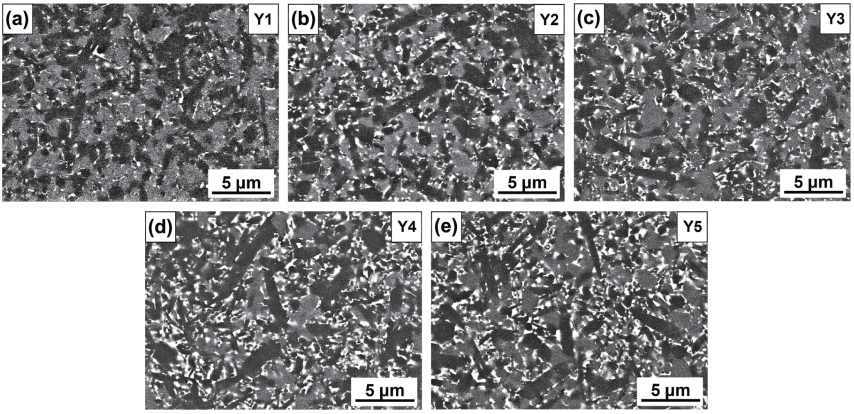
Fig. 8 Microstructure of α/β-SiAlON ceramics with different amounts of excess Y2O3 synthesis additives (m = 1.7, n = 1.0, z = 0.7, synthesized at 1750 ℃) obtained under SEM–BSE mode: samples (a) Y1, (b) Y2, (c) Y3, (d) Y4, and (e) Y5.
To further investigate the intergranular phase, TEM observation was carried out using sample Y3 as a typical sample, and EDX analysis was applied for qualitatively analyzing the composition, as shown in Fig. 9. Through the diffuse diffraction beam, the triple grain boundary junction in Fig. 9(b) was confirmed to be glass phase, and the dark area in Fig. 9(c) was confirmed to be crystallite phase (M'SS). Detailed EDX analysis in Figs. 9(d) and 9(e) indicated that M'SS phase possessed higher Y and O contents (Y: 8.38 at%, O: 13.75 at%) than those of glass phase (Y: 3.14 at%, O: 4.89 at%). This explained the phenomenon that high amount of excess Y2O3 (> 3 wt%) promoted the crystallization level of M'SS phase (samples Y4 and Y5 in Fig. 7).
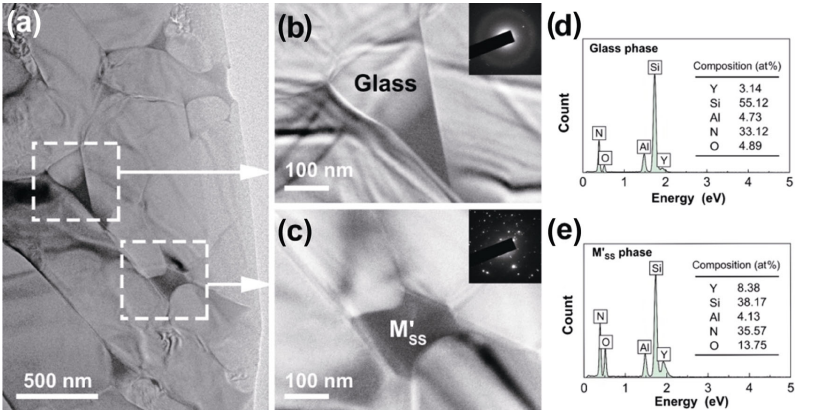
Fig. 9 TEM images and EDX analysis of sample Y3: (a) low-magnified morphology, (b) glass phase (triple junction), (c) M'SS phase, (d) EDX data of glass phase, and (e) EDX data of M'SS phase.
Figure 10 shows the bulk density, hardness, and fracture toughness variations with the amount of excess Y2O3. The bulk density of the α/β-SiAlON ceramics gradually increased from 3.23 to 3.29 g·cm−3 with the increase of excess Y2O3. This could be mainly due to the high density of Y2O3 (5.01 g·cm−3), and moderate improvement of densification, though near full stage, was achieved. Moreover, the hardness barely changed with the increase in the amount of excess Y2O3 from 1 to 3 wt% (16.48–16.59 GPa) since the content of α phase was similar in samples Y1–Y3 (29%–34%), whereas the fracture toughness gradually enhanced from 4.09±0.10 to 4.59±0.24 MPa·m1/2 due to the elongated β grain growth (Fig. 8). When the amount of excess Y2O3 was increased to 4 and 5 wt%, owing to the substantial crystallization of M'SS phase, the hardness evidently reduced to 15.71±0.12 and 15.62±0.08 GPa, respectively, and the fracture toughness declined to 3.76±0.10 and 3.66±0.17 MPa·m1/2, respectively.
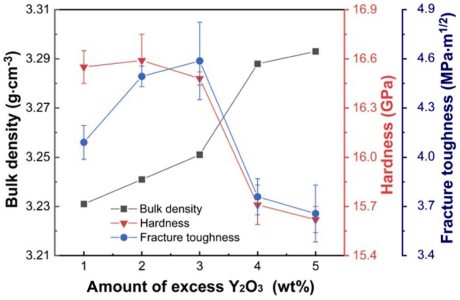
Fig. 10 Bulk density, hardness, and fracture toughness variations with the change in the the amount of excess Y2O3.
3. 4 Machining test
The wear resistance of microwave-synthesized α/β-SiAlON ceramic cutting inserts was studied by rough milling Inconel 718 superalloy and the results were compared with the commercial α/β-SiAlON ceramic cutting insert (Kennametal KYS30). Figure 11 shows the machined surface of the Inconel 718 superalloy. The distance between the cutting traces was close to the feed rate (fz = 0.12 mm) of the cutting insert. Furthermore, owing to the high cutting force of rough milling, the edge of the cutting trace was uneven and some parts even got torn. The roughness (Ra) of the machined surface was measured at 3.27±0.67 μm, which met the requirement of rough milling (6.3 μm).
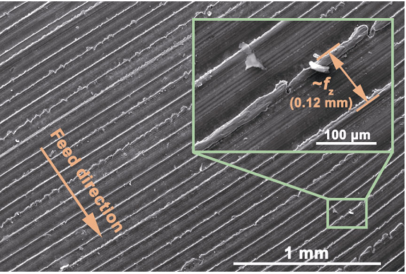
Fig. 11 Machined surface of Inconel 718 superalloy.
Figure 12 exhibits the comparative analysis of the topographies of flank face of microwave-synthesized and commercial α/β-SiAlON ceramic cutting inserts (Y1–Y5, KYS30) under different removed material volumes (RMVs), and the corresponding maximum flank wear (VB) is shown in Fig. 13. Sample Y1 was obtained with the shortest tool life, and the spalling occurred at the initial stage (RMV = 7.43 cm³) and the flank wear increased sharply with the continuation of the machining process. This phenomenon could be due to its poor density (Fig. 9), small aspect ratio, and grain size of β grains (Fig. 8(a)); as a result, the cracks extended easily from the pores. With increasing amount of excess Y2O3 to 2 and 3 wt%, the wear resistance of samples Y2 and Y3 was well improved due to the enhanced densification, elongated β grains, and improved mechanical properties. Notch wear was observed to be the main wear mechanism, while no evident spalling and fracture was observed in the flank face. For samples Y4 and Y5, their wear resistance was obviously declined as compared with samples Y2 and Y3 because their mechanical properties were poor (Fig. 9), but it was not expected to be catastrophic (better than Y1 and close to KYS30). This was probably owing to the increased crystallinity and coalescence of the intergranular phase that reduced the chemical reaction tendency of α/β-SiAlON cutting inserts [19], and the elongated and coarsened β grains resulted from the high excess Y2O3 amount (Fig. 8). Fracture was observed for sample Y5 at the final stage (RMV = 37.13 cm³), which became the main failure. This could have resulted from the high cutting force and its low fracture toughness. The wear mechanism of commercial insert KYS30 was similar to those of samples Y2 and Y3; however, it suffered more severe wear under the same cutting conditions, and the tool life was shorter. As shown in Fig. 13, sample Y3 was obtained with the optimal wear resistance, whose tool life increased by approximately 75% compared to that of commercial insert KYS30 (1.5 mm was set as the allowable VB).
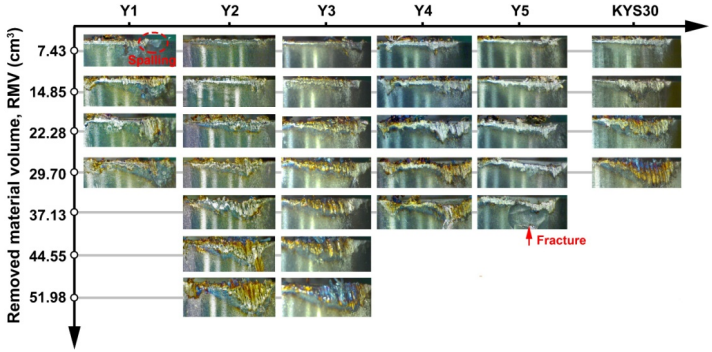
Fig. 12 Flank face morphologies of microwave-synthesized α/β-SiAlON samples (Y1–Y5) and commercial α/β-SiAlON insert (KYS30) under different RMVs.
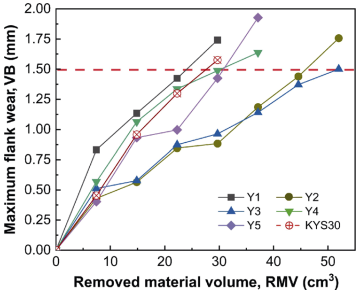
Fig. 13 Corresponding maximum flank wear measured from Fig. 12.
Figure 14(a) shows the worn morphologies of the flank face of sample Y2 at final stage (RMV = 51.98 cm³). Severe adhesion was observed on the most worn area of the insert, while some chipping occurred at the tool edge, indicating that the adhesive wear was the dominant wear mechanism. This phenomenon could be attributed to the large cutting force and high chemical activity of Inconel 718 superalloy, which resulted in the notch in the flank face and build-up edge (BUE) in the tool edge. Moreover, the insert (Y2) was vertically cut through the position of maximum flank wear, and the polished cross-sectional morphology (A–A view) is shown in Fig. 14(b). From the EDX mapping of the selected area, the adhesive layer of Inconel 718 with depth over 50 μm and tool substrate can be clearly distinguished. Cracks were found to extend along the interface of the insert, adhesive layer, and tool substrate, illustrating that the bonding was not stable. During high-speed rough machining, adhesive layer was ground out, causing further wear, followed by the adhering of the new layer on the worn area.
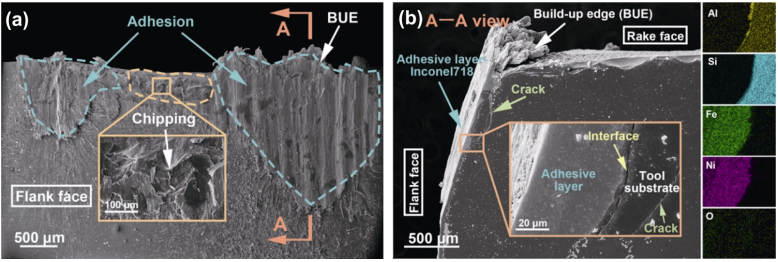
Fig. 14 SEM images of worn morphologies of sample Y2 (RMV = 51.98 cm³): (a) flank face, (b) cross-sectional morphology (A–A view), and corresponding EDX mapping of selected area.
Figure 15 shows the microstructure of microwave-synthesized sample Y3 and commercial insert KYS30, and further comparison of other factors is presented in Table 3. Both samples Y3 and KYS30 were obtained with uniform morphologies of α-SiAlON, β-SiAlON, and intergranular phase. However, the grain size in sample Y3 was much more coarsened than that of sample KYS30. Table 3 presents that the cation type of KYS30 was detected as Yb whose density was higher than that of Y, and thus the density of KYS30 was relatively higher than that of sample Y3. In addition, the intergranular phase of the two samples was different, that is, M'SS phase was detected in sample Y3, while that in KYS30 amorphous phase was detected. KYS30 was measured with lower α/(α+β) ratio (18%) than sample Y3 (34%). Consequently, KYS30 possessed lower hardness and higher fracture toughness; however, the difference was slight. In this stage, the improved tool life of sample Y3 could be attributed to the crystallization of intergranular phase and coarsened grain size [35,36].
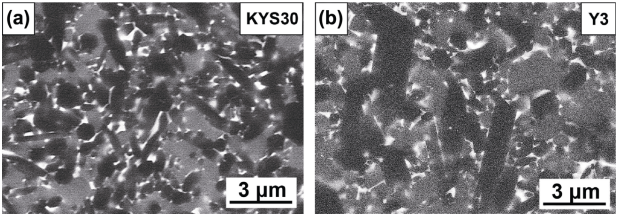
Fig. 15 Microstructure of commercial and microwave-synthesized α/β-SiAlON ceramic cutting inserts obtained under SEM–BSE mode: (a) KYS30 and (b) Y3.
Table 3 Comparison of commercial and microwave-synthesized α/β-SiAlON milling insertsc

4 Conclusions
In this study, duplex α/β-SiAlON ceramic cutting inserts with nominal composition of α:β = 30:70 were successfully fabricated by microwave synthesis. The solid solution parameters (m, n, z) and synthesis temperature were optimized by orthogonal experiment, and the effects of z value and amount of excess Y2O3 synthesis additive on phase assemblage, microstructure, mechanical properties, and wear resistance were systematically investigated. The main conclusions drawn from this study are as follows:
1) The increase in m value from 1.0 to 1.7 could promote the formation of α phase (8.3% to 25.7%). thus improving the hardness. Moreover, the high synthesis temperature could promote the solid solution reaction, densification, and hardness.
2) α + liquid → β + M'SS + 21R transformation reaction was observed when z value exceeded 1.0 under synthesis temperature of 1750 ℃. The microstructure analysis indicates that the area of β-SiAlON phase noticeably increased when z value exceeded 1.0, and the β grain growth was relatively accelerated with increasing z value. The hardness and fracture toughness of α/β-SiAlON ceramics exhibited an overall declining trend with increasing z value, and the sample with z value of 1.14 acquired the worst mechanical properties due to the substantial crystallization of intergranular phase.
3) Increasing excess Y2O3 amount could improve the grain growth and aspect ratio of the β-SiAlON grains and enhance the densification. However, the excess Y2O3 over 4 wt% led to the substantial crystallization of M'SS phase and decline in the mechanical properties and wear resistance of α/β-SiAlON ceramics.
4) Adhesive wear was the dominant wear mechanism of α/β-SiAlON ceramic cutting inserts in rough milling Inconel 718 superalloy, and the wear resistance of α/β-SiAlON ceramic cutting insert was found to be related with the mechanical properties, microstructure, and intergranular phase.
5) The microwave-synthesized α/β-SiAlON cutting insert with modified parameters (m, n, z values and excess Y2O3 of 1.7, 1.0, 0.7, and 3 wt%, respectively, synthesized at 1750 ℃) was obtained with the optimal comprehensive properties, whose tool life was increased by approximately 75% compared to commercial α/β-SiAlON cutting insert.
References: Omitted
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.